Observing the Development of Quinolones from the Rational Application of Antibacterial Drugs During the COVID-19 Pandemic

Professor Yang Wenjie
Chief Physician, Professor, Mentor to Graduate Students. Director of Department of Infectious Diseases, Tianjin First Central Hospital. Member of the Infection Working Group of the National Health Commission’s Antimicrobial Drug Clinical Application and Bacterial Resistance Evaluation Committee, Member of the National Health Commission’s Hospital Infection Control Standards Professional Committee, Member of the National Health Commission’s Medical Institution Infection Prevention and Control Expert Committee, Vice Chairman of the Infectious Disease Evidence-Based and Transformation Committee of the Chinese Research Hospital Association, Member of the Standing Committee of the Branch of Bacterial Infection and Drug Resistance Prevention and Control of the Chinese Medical Doctor Association, Chairman of Tianjin Municipal Committee, Chairman of Tianjin City, Member of Infectious Disease Physician Branch of Chinese Medical Doctor Association, President of the Association of Infectious Disease Physicians of Tianjin Medical Doctor Association, Chairman of Infectious Disease Professional Committee of Tianjin Association of Integrative Traditional Chinese and Western Medicine, Director of the Office of the Expert Committee on the Rational Application of Antibacterial Drugs of the Tianjin Municipal Health Commission, Director of the Quality Control Center for Infectious Diseases, Director of the Quality Control Center for Fever Clinics, Member of the Standing Committee of the Rational Application of Antimicrobial Drugs Committee of the Chinese Hospital Association, Member of the Infectious Diseases Branch of the Chinese Medical Doctor Association, Vice Chairman of Tianjin Municipality, Member of the Standing Committee of the Hospital Infection Management Quality Control Branch of the Chinese Geriatrics Association, Vice Chairman of Hospital Infection Control Branch of Tianjin Preventive Medicine Association. Editorial board member for “Chinese Journal of Clinical Infectious Diseases,” “Adverse Drug Reactions Journal,” “Journal of Clinical Hepatology” and other journals, Reviewer of “Chinese Journal of Epidemiology,” “Infectious Diseases & Immunity,” “Chinese Journal of Pharmacovigilance” and other journals.
Introduction: Quinolone antibacterial drugs have been refined and iterated. Due to their advantages such as broad antibacterial spectrum, good bioavailability, and wide tissue distribution, they have become one of the most widely used antibacterial drugs in clinical practice.
At present, quinolone antibiotics (referred to as “quinolones”) are widely used in the treatment of various systemic infections. Since the first-generation drug was launched in 1962, quinolones have gone through more than half a century of iterative history before their importance in clinical anti-infective therapy was finally established, which is owing to their broad antibacterial spectrum, outstanding antibacterial activity, high bioavailability, few adverse reactions, wide tissue distribution and long half-life.
In recent years, the fluctuations of the COVID-19 pandemic and viral infections have severely affected the respiratory tract of patients. Antibacterial treatment of patients with COVID-19 combined with secondary bacterial infections has also become a topic of clinical concern. For this reason, “Medical World” sincerely invited Professor Yang Wenjie from Tianjin First Central Hospital to discuss the clinical application of quinolones during the pandemic.
Q1. Quinolones have become one of the most widely used antibacterial drugs in clinical practice, following long-term development since the first generation of quinolones came into the market. Combining antibacterial spectrum, drug susceptibility data, pharmacokinetics/pharmacodynamics (PK/PD) and clinical application experience, could you please explain the characteristics and differences of the classic or traditional/new fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin, sitafloxacin) in the field of respiratory tract infection and other applications?
Quinolones have been on the market for more than 60 years. The first-generation quinolone, which is represented by nalidixic acid, has a narrow antibacterial spectrum. The second-generation quinolone, while having expanded antibacterial spectrum, is still generally similar to the first-generation quinolone, and is mainly used to treat urinary tract infections (UTIs) and intestinal infections. At present, the new quinolones commonly used in clinical practice, such as ciprofloxacin, levofloxacin, moxifloxacin, sitafloxacin, etc., have already demonstrated a variety of clinical therapeutic advantages.
Compared with the previous quinolones, the new quinolones have the following characteristics: a wider antibacterial spectrum, stronger antibacterial activity, wider distribution in the body, and better PK/PD parameters. They also have high oral bioavailability and low protein binding rates (drugs with high protein binding rate may affect the clinical efficacy in infected patients), and most of them have intravenous and oral dosage forms which are convenient for intravenous/oral sequential treatment. In addition, except for moxifloxacin, they are mainly excreted by the kidneys, so they are not only suitable for respiratory system infection, abdominal infection, etc., but also commonly used in the anti-infection treatment of the urinary system.
Ciprofloxacin – old but still going strong: Ciprofloxacin, as a classic old fluoroquinolone, has poor antibacterial activity against Streptococcus pneumoniae, and poor antibacterial activity against atypical pathogens such as Mycoplasma pneumoniae and Chlamydia pneumoniae, so it is not suitable for the treatment of community-acquired pneumonia (CAP). However, owing to its significant therapeutic effect on pneumonia caused by Pseudomonas aeruginosa, it is more clinically used to treat hospital-acquired pneumonia (HAP). At the same time, ciprofloxacin is also the first drug that was used to treat non-Tuberculous mycobacteria (NTM).
Moxifloxacin – the showstopper: In addition to moxifloxacin’s outstanding antibacterial activity against common pathogens of CAP (Streptococcus pneumoniae, Haemophilus influenzae, and atypical pathogens such as Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila) and anaerobic bacteria such as Bacteroides fragilis, which is common in aspiration pneumonia, it also has good antibacterial effects on community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). At the same time, it is also the “strong antibacterial agent” of Stenotrophomonas maltophilia (a common pathogen of HAP and ventilator-associated pneumonia [VAP]) and is also commonly used in the treatment of drug-resistant tuberculosis and some NTMs.
Levofloxacin – the average performer: Levofloxacin takes into account the antibacterial activities of the above two drugs, so it is useful in both CAP and HAP, but the antibacterial activities against Pseudomonas aeruginosa and Streptococcus pneumoniae are weaker than those of ciprofloxacin and moxifloxacin. In terms of safety, levofloxacin has less risk of hepatic damage and less impact on cardiac QT interval, and is better tolerated. The internationally recognized dosage of levofloxacin is 750mg qd. In contrast, the lower dosage typically used in China limits its maximum efficacy.
Sitafloxacin – the up-and-coming youngster: Sitafloxacin, the new quinolone with a wider antibacterial spectrum, was launched in China in 2019. Clinically, it has antibacterial activity against Gram-positive cocci, Gram-negative bacilli, aerobic bacteria, anaerobic bacteria, drug-resistant bacteria and some atypical pathogens. Indications for sitafloxacin include UTIs, intestinal infection, respiratory tract infection and various infectious diseases related to gynecology, ENT, dentistry, and dermatology. Owing to the cis-fluorocyclopropylamine group contained in the molecular formula, sitafloxacin not only has good pharmacokinetic properties, but also stronger in vitro antibacterial activity than most quinolones, which can effectively reduce the incidence of adverse reactions in patients.
Data from both Japan and China suggest that sitafloxacin has overall stronger antibacterial activity than previous quinolones. It is effective against gram-positive cocci (including aerobic bacteria such as penicillin-resistant Streptococcus pneumoniae and oxacillin-resistant Staphylococcus aureus, and anaerobic bacteria such as Peptostreptococcus), gram-negative bacilli (including Enterobacteriaceae such as Escherichia coli, aerobic bacteria such as Acinetobacter baumannii and Pseudomonas aeruginosa, anaerobic bacteria such as Fusobacterium), and atypical pathogens (including mycoplasma, chlamydia, and legionella). Additionally, sitafloxacin has good antibacterial activity and does not easily become ineffective as a result of drug resistance. Studies in China have shown that the sensitivity rate of ciprofloxacin-resistant Escherichia coli to sitafloxacin is as high as 72.5%.
Table 1. Susceptibility (S) and resistance (R) of Pseudomonas aeruginosa to antibacterial drugs 1
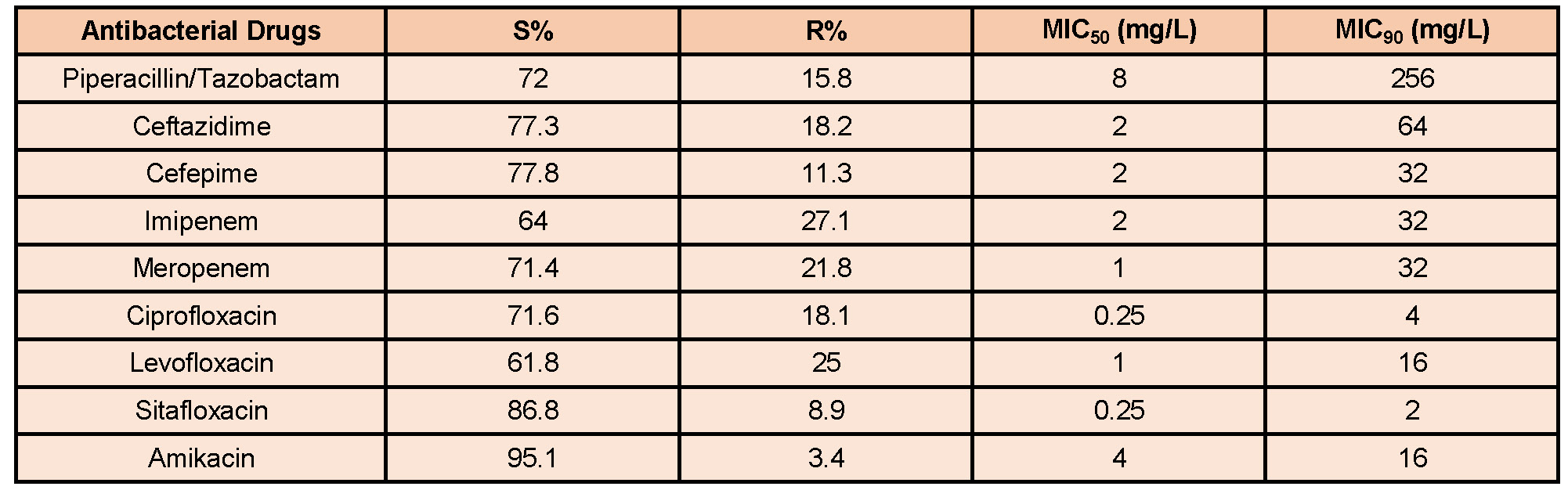
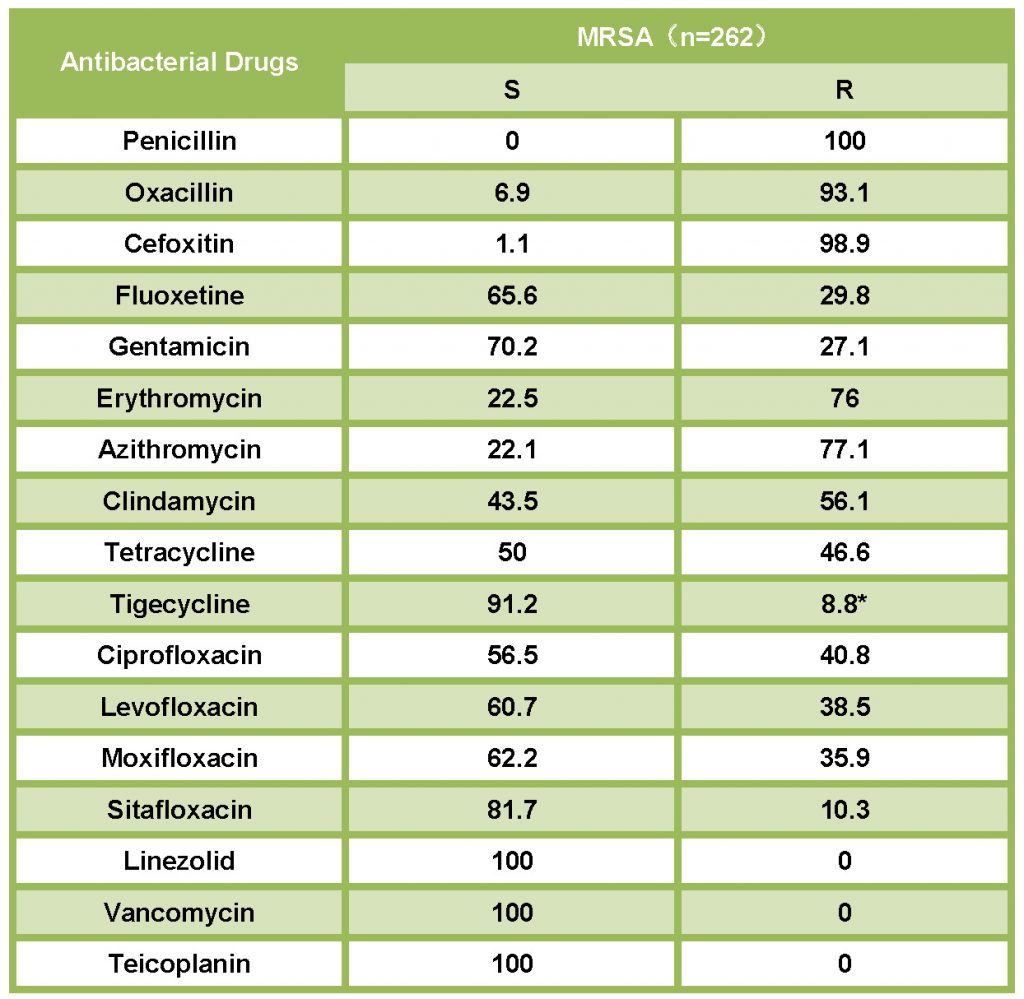
Table 2. Sensitivity and resistance of MRSA to antibacterial drugs1
Q2. Enterobacteriaceae are the main pathogens of genitourinary system infections. Could you please talk about the clinical advantages of different fluoroquinolones in the treatment of genitourinary infections by combining the respective characteristics of classic traditional/new fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin, sitafloxacin)? What are the differences between them?
The pathogenic bacteria of genitourinary tract infections are mainly Enterobacteriaceae bacteria (such as Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus, etc.), but Pseudomonas aeruginosa infection can also occur in some patients with stones, obstruction, and indwelling implant devices. In addition, enterococcal UTIs may occur in some female patients. At present, the drug resistance rate of Escherichia coli to ciprofloxacin and third-generation cephalosporins exceeds 50%, and the drug resistance of community pathogens causing UTIs is also relatively common.
Upper and lower UTIs infections cannot be treated equally. Quinolones are concentration-dependent antibacterial drugs which can achieve good antibacterial effects, even in the case of drug resistance, and can be used to treat lower UTIs such as cystitis and urethritis because of a high concentration in urine. The treatment of upper UTIs are not limited by this need, because the kidneys are rich in blood supply. In addition to considerations around the concentration of antibacterial drugs in urine, the antibacterial treatment should also ensure sufficient blood drug concentration to prevent patients from suffering from urogenous sepsis.
Tissue concentrations and renal excretion rates are key considerations in the treatment of UTIs. Ciprofloxacin, levofloxacin, moxifloxacin, and sitafloxacin have different renal metabolism ratios: the renal metabolism rate of ciprofloxacin oral preparations is 40–50%, and for intravenous preparations are 50–70%. The renal metabolism rate of tafloxacin after oral administration is 78%, that of levofloxacin is 80–86%, and that of moxifloxacin is only 20%. Therefore, moxifloxacin is not usually used as an antibacterial choice for UTIs, whereas ciprofloxacin, levofloxacin, and sitafloxacin should be the preferred choice.
In recent years, the problem of drug resistance of pathogenic bacteria has been of concern widely. If the pathogenic bacteria detected in patients with upper UTIs is Escherichia coli, ciprofloxacin and levofloxacin are usually not the first choice for empiric treatment, because Escherichia coli strains are generally resistant to these two classes of drugs. That is, unless the urine culture results show that the pathogenic bacteria are sensitive to the drugs. In contrast, sitafloxacin has a good effect on Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae (including extended-spectrum β-lactamase [ESBL]-producing strains), and so on. Even for quinolone-resistant Escherichia coli which, owing to multiple mechanisms such as active efflux and gene mutations of gyrA, gyrB, parC, and parE, which cause changes in membrane permeability, sitafloxacin can still effectively exert antibacterial activity, and therefore is the preferred solution for the clinical treatment of UTIs.
Q3. Respiratory quinolones and β-lactam antibiotics are commonly used clinically when patients with COVID-19 have indications for antimicrobial therapy. Please talk about the clinical application value of respiratory quinolones (levofloxacin, moxifloxacin, sitafloxacin) for patients in different treatment sites based on your personal clinical experience.
In recent years, the continuous spread and transmission of the new coronavirus variants has led some patients to become prone to bacterial infections and pneumonia owing to COVID-related damage to the respiratory tract and alveolar epithelium, impaired bronchial clearance function, and impaired cellular immunity. In terms of co-infected pathogens, the pathogenic bacteria detected in young CAP patients with new-onset coronavirus pneumonia are similar to the general CAP pathogens, while elderly CAP patients with underlying diseases are more commonly infected with Enterobacteriaceae such as Klebsiella pneumoniae. During hospitalization, nosocomial-acquired infections easily occur especially among patients with severe illness, multiple complications, and in those unable to take care of themselves. HAP/VAP is the main infection that occurs; other infections include UTIs, catheter-related bloodstream infection, skin and soft tissue infection, etc.
Before initiating anti-infection treatment, it should be determined whether the patient has a simple new coronavirus infection or a new coronavirus combined bacterial infection. In addition to the conventional total white blood cell count, neutrophil ratio, and lymphocyte count, procalcitonin (PCT) can also be used as a reference index. Bacterial infection is usually not considered in patients with PCT<0.05, but patients with moderately severe new coronavirus infection confirmed by Curb-65 score or Pneumonia Severity (PSI) score evaluation with severe underlying diseases should be considered as having bacterial infection when PCT>0.1. When this occurs, etiological examination should be conducted and antibiotics should be used rationally. The drug withdrawal time should be planned by monitoring the changes of PCT indicators after taking the drug.
For patients with 2019 novel coronavirus (2019-nCoV) infection who are being considered to have bacterial pneumonia, treatment options must be carefully selected owing to their complex conditions. For non-severely ill patients in general wards, antibiotics that can cover atypical pathogens should be selected for treatment, whereas severe patients admitted to ICU should be empirically covered for MRSA. Respiratory quinolones (levofloxacin, moxifloxacin, sitafloxacin) are more suitable for these patients owing to the low bioavailability of first and second-generation cephalosporin oral preparations (cephalexin and cefaclor, etc.) and the generally high bacterial resistance rate. These agents have become a better choice for the treatment of CAP in coronavirus combined with bacterial infection.
HAP is mainly caused by Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus aureus. For patients with underlying diseases, we should be more vigilant about the risk of specific pathogen infection and drug resistance. For example, COVID-19 patients with a history of diabetes have a higher risk of methicillin-resistant Staphylococcus aureus (MRSA) infection. In addition, owing to the increased frequency of corticosteroid application in patients with coronavirus, the risk of Pseudomonas aeruginosa infection has also increased. Sitafloxacin is superior to other quinolones for sensitive bacteria such as Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. For carbapenem-resistant Acinetobacter baumannii HAP, a combination therapy such as sitafloxacin and colistin can be attempted. Moreover, because most quinolones have oral dosage forms, sequential treatment can be better provided, which is beneficial to shorten the hospitalization time of patients. In general, quinolone antibacterial drugs have been continuously updated and iterated, providing us with a better choice of quinolone treatment options in clinical practice, and bringing the greatest benefits to patients with better clinical efficacy.
References:
1. Li Yun, Zheng Bo, Lu Yuan, Xue Feng, Zhang Xiuzhen, Hu Yunjian, Jin Yufen, Hu Zhidong, Zhao Jianhong, Pan Shiyang, Li Huayin, Yu Yunsong, Li Yan, Liu Wenen, Andy Lau, Fei Ying, Liu Zhiyong, Xu Xiuli, Pei Fengyan, Meng Ling, Ji Ping, Tang Jin, Fu Huiqun, Zhu Lei, Wang Shan, Zhang Jia, Wang Qing, Liu Yaoyao. China Antimicrobial Resistance Surveillance Trial (CARST) Program, 2019-2020 Gram-Negative Bacilli Surveillance Report[J]. The Chinese Journal of Clinical Pharmacology, 2022,38(05):432-452.










