CRAVIT

The product information provided in this page is intended only for the selected country.
Cravit contains levofloxacin as an active ingredient, and is used to treat a variety of bacterial infections. This medication belongs to a class of drugs known as quinolone antibiotics and works by inhibiting the growth of bacteria. It is usually used to treat a range of infections including skin, respiratory, urinary tract, gynaecologic and otologic infections.
Cravit tablets are indicated for the treatment of adults (≥ 16 years of age) with mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed as follows: Acute sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae or Moraxella catarrhalis.
Acute exacerbation of chronic bronchitis due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae or Moraxella catarrhalis.
Community-acquired pneumonia due to Staphylococcus aureus, Streptococcus pneumoniae (including penicillin-resistant strains), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella pneumophila or Mycoplasma pneumonia.
Nosocomial pneumonia due to methicillin-susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae or Streptococcus pneumoniae. Adjunctive therapy should be used as clinically indicated. Where Pseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an anti-pseudomonal β-lactam is recommended.
Uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma and wound infections due to Staphylococcus aureus and Streptococcus pyogenes.
Complicated skin and skin structure infections (mild to moderate) due to methicillin-susceptible Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes or Proteus mirabilis.
Urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa or Staphylococcus saprophyticus.
Chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis or Staphylococcus epidermidis.
Pyelonephritis (mild to moderate) caused by Escherichia coli.
Empirical treatment for community acquired pneumonia most likely caused by organisms susceptible to levofloxacin.
Third-line therapy for Helicobacter pylori infection: Levofloxacin-based Triple Therapy: Levofloxacin, in combination with other antimicrobial agent and proton-pump inhibitor as triple therapy, is indicated for the treatment of patients with gastric ulcer caused by Helicobacter pylori infection and doudenal ulcer disease.
Empirical Treatment for Community-Acquired Pneumonia: 500 or 750 mg is administered once daily.
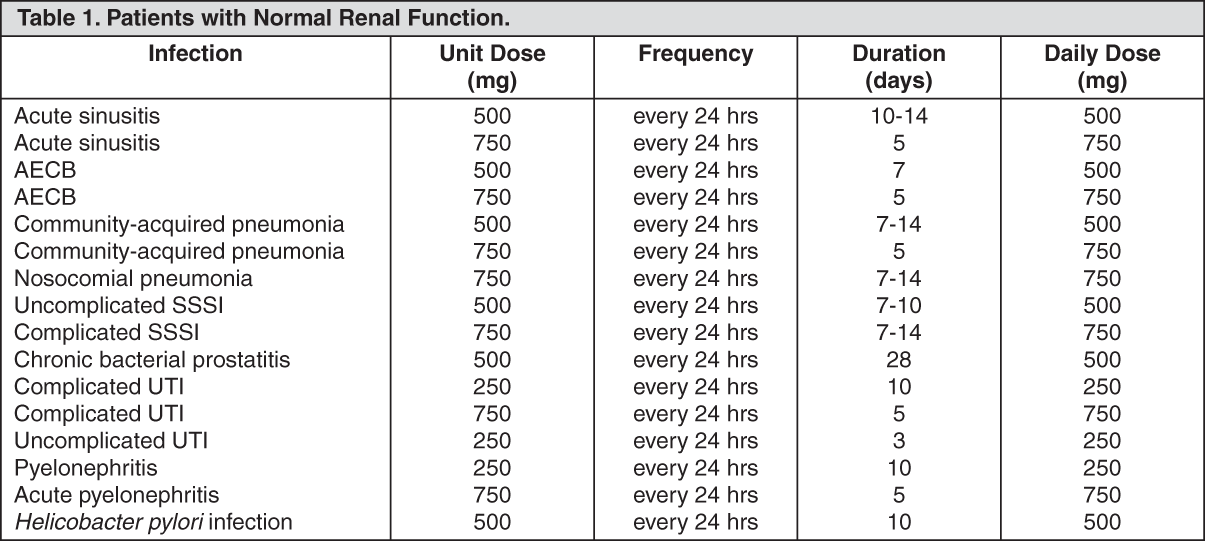
Patients with Normal Renal Function: See Table 1.
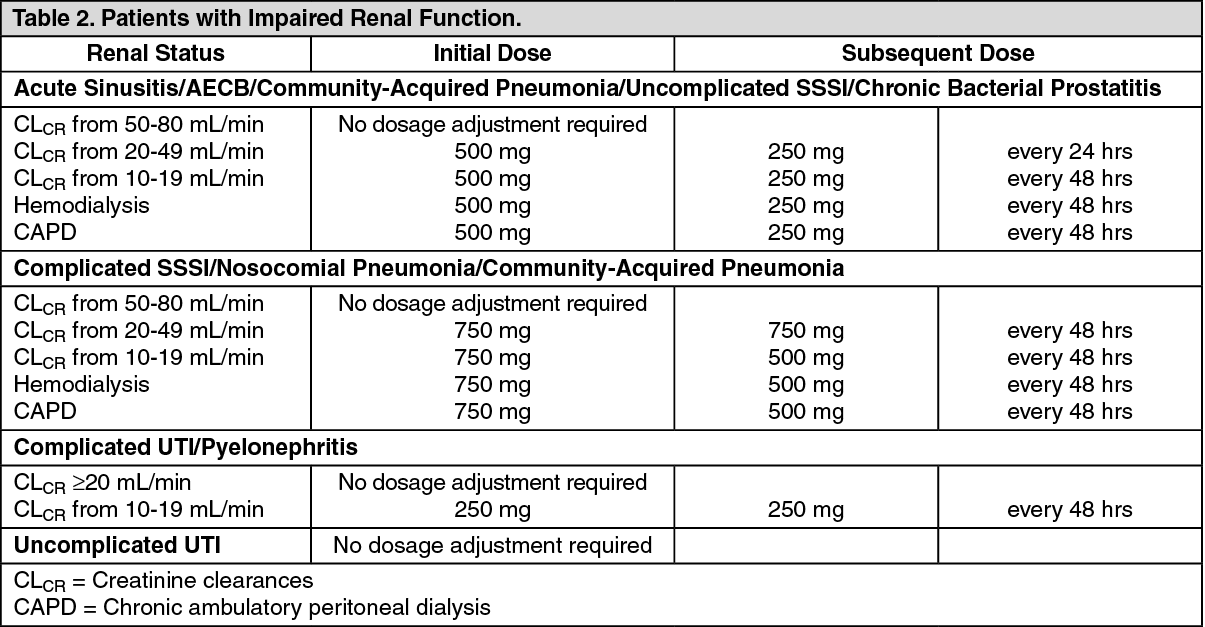
Patients with Impaired Renal Function: See Table 2.
When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.

The serum creatinine should represent a steady state of renal function.
Patients with Impaired Liver Function: No adjustment of dosage is required since levofloxacin is not metabolized to any relevant extent by liver and is mainly excreted by kidneys.
Elderly: No adjustment is required in the elderly, other than that imposed by consideration of renal function.
According to toxicity studies in animals, the most important signs to be expected following acute overdosage of Cravit 500 mg or 750 mg are central nervous system symptoms such as confusion, dizziness, impairment of consciousness, and convulsive seizures, as well as gastrointestinal reactions such as nausea and mucosal erosions.
In the event of an acute overdosage, the stomach should be emptied. Antacid may be used for protection of gastric mucosa. No specific antidote exists. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Levofloxacin is contraindicated in the following patients: Patients with a history of hypersensitivity to levofloxacin, ofloxacin or any excipients of this product.
Patients with epilepsy.
Patients with history of tendon disorder related to fluoroquinolones administration.
Children or adolescents below the age of 16 years.
Pregnant women or women suspected of being pregnant.
Breast-feeding women.
Use during pregnancy: Levofloxacin must not be used in pregnant women or women suspected of being pregnant since the safety of the product in pregnant women has not been established (see CONTRAINDICATIONS).
Use during lactation: Since ofloxacin is known to be excreted in breast milk, nursing mothers should be guided to avoid the breast-feeding during treatment with levofloxacin (see CONTRAINDICATIONS).
Empirical Treatment for Community-Acquired Pneumonia: 500 or 750 mg is administered once daily.
Patients with Normal Renal Function: See Table 1.

Patients with Impaired Renal Function: See Table 2.

When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.

The serum creatinine should represent a steady state of renal function.
Patients with Impaired Liver Function: No adjustment of dosage is required since levofloxacin is not metabolized to any relevant extent by liver and is mainly excreted by kidneys.
Elderly: No adjustment is required in the elderly, other than that imposed by consideration of renal function.
Cravit Tab should be administered with caution in the following patients: Patients with severe renal impairment: Levofloxacin is excreted mainly by the kidneys and persistence of high serum level in patients with renal impairment has been reported.
Patients with known or suspected CNS disorder such as epilepsy or with a history of convulsive disease that may predispose to seizures or lower the seizure threshold, convulsion may occur.
Diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (especially, sulfonylureas or with insulin preparations).
Patients with a history of hypersensitivity to quinolone antibiotics.
Patients with serious heart diseases (e.g. arrhythmia and ischemic heart disease) patients with uncorrected electrolyte imbalance (e.g. hypokalemia, hypomagnesemia) and patients receiving class IA and III antiarrhythmic agents. QT prolongation may occur (see SERIOUS ADVERSE REACTION under ADVERSE REACTIONS and INTERACTIONS).
Patients with myasthenia gravis: Levofloxacin may cause exacerbation of myasthenia gravis symptoms.
Levofloxacin is more soluble than other quinolones, adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of highly concentrated urine.
Levofloxacin may inhibit the growth of Mycobacterium tuberculosis, and therefore, may give false-negative results in the bacteriological diagnosis of tuberculosis.
Some undesirable effects (see ADVERSE REACTIONS) may impair the patient’s ability to concentration and react, and therefore may constitute a risk in situation where these abilities are of special importance (e.g. driving a car or operating machinery).
Excessive exposure to sunlight should be avoided. However, phototoxicity has been observed very rare: incidence < 0.01%. Therapy should be discontinued if phototoxicity (e.g. a skin eruption) occurs.
Patients complicated with aortic aneurysm or aortic dissection, or patients who have a previous history, positive family history, or risk factors (Marfan syndrome, etc.) of aortic aneurysm or aortic dissection [The increased risk of aortic aneurysm and dissection after intake of fluoroquinolones have been reported in overseas epidemiologic studies (see SERIOUS ADVERSE REACTION under ADVERSE REACTIONS)].
Aortic aneurysm or aortic dissection may occur; therefore, patients should be carefully observed and instructed to seek medical attention immediately if they experience symptoms, e.g. in case of pain in the abdomen, chest, or back. Imaging assessment should be considered if necessary, for patients complicated with aortic aneurysm or aortic dissection, or patients who have a previous history, positive family history, or risk factors of aortic aneurysm or aortic dissection (see SERIOUS ADVERSE REACTION under ADVERSE REACTIONS).
Effect on ability to drive and use machine: Neurologic adverse effects such dizziness/vertigo and somnolence may occur. Therefore, patients should be instructed that such neurologic adverse effects may impair the patient’s ability to concentrate and react, and therefore may constitute a risk in situation where these abilities are of special importance (e.g. activities at high place, driving a car or operating machinery).
Use in the Elderly: Since renal function is generally depressed in geriatric patients and levofloxacin is excreted mainly by the kidneys, levofloxacin should be used with caution in this population (see PRECAUTION ASSOCIATED WITH DOSAGE AND ADMINISTRATION: GERIATRIC PATIENTS under DOSAGE & ADMINISTRATION).
The following adverse reactions have been reported in clinical studies and post-marketing experience. The incidence identified as follows reflects exposure to 500 mg tablet of levofloxacin in total of 1,930 patients in pooled Phase 3 and Phase 4 clinical trials (i.e., 1,582 patients from Phase 3 clinical trials conducted in Japan (337 patients) and China (1,245 patients) and 348 patients from Phase 4 clinical trials) or 29,880 patients in a post marketing studies conducted in Japan. If the incidence category of an adverse reaction is different between each source (i.e., the incidence from the pooled clinical trials and the incidence from the post-marketing study), the higher frequency is represented.
The following CIOMS frequency rating is used: Very common: 10% ≤ incidence; Common: 1% ≤ incidence <10%; Uncommon: 0.1% ≤ incidence <1%; Rare: 0.01% ≤ incidence <0.1%; Very rare: incidence <0.01%.
*: see Serious Adverse Reaction in the following text. Each incidence is based on serious reactions.
Blood and lymphatic system disorders: Common: anemia.
Very rare: thrombocytopenia*.
Incidence unknown: pancytopenia*, agranulocytosis*, hemolytic anemia with hemoglobinuria*.
Immune system disorder: Incidence unknown: anaphylactoid reaction*.
Metabolism and nutrition disorder: Uncommon: anorexia.
Incidence unknown: hypoglycemia (hypoglycaemic coma may occur)*, hyperglycemia*.
Psychiatric disorders: Common: sleep loss.
Rare: hallucination.
Incidence Unknown: Psychiatric symptoms eg, confusion*, delirium*, depression*.
Nervous System Disorders: Common: dizziness/vertigo, headache.
Uncommon: somnolence, numbness, tremor, mental dullness, dysgeusia.
Rare: consciousness disturbed.
Very Rare: convulsion*, ageusia.
Incidence unknown: peripheral nerve disorder, extrapyramidal disorder, anosmia, parosmia.
Eye disorders: Rare: abnormal vision.
Ear and Labyrinth Disorders: Uncommon: tinnitus.
Incidence unknown: hearing losses.
Cardiac disorders: Uncommon: palpitations.
Incidence unknown: ventricular tachycardia (including Torsades de pointes)*, QT prolonged*, tachycardia.
Vascular disorders: Very rare: shock*. Incidence unknown: hypotension.
Respiratory, thoracic and mediastinal disorders: Uncommon: dry throat.
Incidence unknown: interstitial pneumonia*, eosinophilic pneumonia*.
Gastrointestinal disorders: Common: nausea, vomiting, diarrhea, abdominal discomfort.
Uncommon: abdominal pain, dyspepsia, abdominal distention, constipation.
Rare: stomatitis. Very rare: glossitis.
Incidence unknown: colitis with bloody stool, such as pseudomembranous colitis*.
Hepatobiliary Disorders: Uncommon: hepatic function abnormal (severe hepatic function disorder* may rarely occur.
Incidence unknown: hepatitis fulminant hepatitis*, jaundice*.
Skin and subcutaneous tissue disorders: Uncommon: pruritus, rash.
Rare: hyperhidrosis, urticaria.
Very rare: photosensitivity.
Incidence unknown: toxic epidermal necrolysis (TEN)*, oculomucocutaneous syndrome (Stevens-Johnson syndrome)*, hypersensitivity vasculitis*.
Musculoskeletal and connective tissue disorders: Uncommon: arthralgia, pain in extremity, back pain, weakness.
Rare: arthropathy, myalgia.
Incidence unknown: rhabdomyolysis*, tendon disorders such as Achilles tendonitis or tendon rupture*, exacerbation of myasthenia gravis*, muscle rupture.
Renal and urinary disorders: Uncommon: hematuria, urinary distention.
Rare: pollakiuria, oliguria, acute renal failure*.
Incidence unknown: interstitial nephritis*, anuria, dysuria.
General disorders and administration site conditions: Uncommon: thirst, chest discomfort, malaise, feeling hot, edema.
Very Rare: pyrexia.
Incidence unknown: chest pain.
Investigations: Common: AST increased, ALT increased, LDH increased, white blood cell count decreased, eosinophil count increased.
Uncommon: creatinine increased, urinary protein positive, alkaline phosphatase increased, γ-GTP increased, blood bilirubin increased, lymphocyte count decreased, neutrophil count decreased, CPK increased, glucose urine present, blood lucose decreased, platelet count decreased.
Rare: BUN increased, urine output decreased.
Very Rare: blood glucose increased.
*See Serious Adverse Reaction in the following text.
Serious Adverse Reaction: The following serious adverse reactions have been reported in patients receiving therapy with levofloxacin. If the following reactions are suspected, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measure should be taken: Shock or anaphylactoid reaction (initial symptoms: erythema, rigor, dyspnea, etc.).
Toxic epidermal necrolysis (TEN) or oculomucocutaneous syndrome (Stevens-Johnson syndrome).
Convulsion.
QT prolonged and ventricular tachycardia (including Torsades de pointes): During post-marketing surveillance, prolonged QT which may sometimes lead to the occurrence of ventricular tachycardia including torsades de pointes have been reported spontaneously in patients taking levofloxacin. The risk of the events may be increased in patients with serious heart diseases (e.g. arrhythmia and ischemic heart disease), patients with uncorrected hypokalemia, patients receiving Class IA (quinidine sulfate, procainamide hydrochloride) and Class III (amiodarone hydrochloride, sotalol hydrochloride) antiarrhythmic agents and in geriatric patients.
Acute renal failure or interstitial nephritis.
Hepatitis fulminant, hepatic function disorder or jaundice (initial symptoms: nausea, vomiting, anorexia, malaise, pruritus, etc.).
Pancytopenia, agranulocytosis (initial symptoms: pyrexia, pharynx pain, malaise, etc.), hemolytic anemia with hemoglobinuria or thrombocytopenia.
Interstitial pneumonia or eosinophilic pneumonia accompanied with pyrexia, cough, dyspnea, abnormal chest X-ray, or eosinophilia, etc.
Serious colitis with bloody stool, such as pseudomembranous colitis: If such symptoms as abdominal pain and frequent diarrhea are noted, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measures taken.
Rhabdomyolysis characterized by myalgia, weakness, elevated CK (CPK) and increased myoglobin in plasma and urine, etc., and accompanied with acute exacerbation of renal function.
Dysglycemia: During post-marketing surveillance, hypoglycemia and hyperglycemia have been reported in patients taking levofloxacin. Serious symptoms such as hypoglycemic coma have been reported in patients receiving levofloxacin. Hypoglycemia may be prone to develop in patients with diabetes mellitus (especially, those receiving sulfonylureas or insulin preparations), patients with impaired renal function and geriatric patients.
Tendon disorders such as Achilles tendonitis or tendon rupture: If symptoms such as pain and edema in the peritendinous region are observed, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measures taken. The risk of tendonitis and tendon rupture is increased in those over age 60, in those on concomitant corticosteroid therapy, and transplant recipients.
Psychiatric symptoms such as confusion, delirium and depression.
Hypersensitivity vasculitis: If symptoms such as pyrexia, abdominal pain, arthralgia, purpura or maculopapules, and skin biopsy evidence of leukocytoclastic vasculitis are observed, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measures taken.
Exacerbation of myasthenia gravis.
Patients received Levofloxacin dose of 750 mg may develop some adverse reaction such as dizziness, headache, nausea or vomiting more than Levofloxacin dose of 500 mg.
Aortic aneurysm, aortic dissection (incidence unknown): Aortic aneurysm or aortic dissection may occur. If any abnormalities are observed, appropriate medical treatment should be taken (see Precautions).
Antacid, Sucralfate, Metal Cations, Multivitamins: While the chelation by divalent cations is less marked than with other quinolones, concurrent administration of levofloxacin with antacids containing magnesium or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least two hours before or two hours after levofloxacin administration.
Theophylline, Fenbufen or similar non-steroidal anti-inflammatory drugs (phenylacetic acid/propionic acid derivatives): No pharmacokinetic interactions of levofloxacin were found with theophylline in a clinical study. However there are indications of a pronounced lowering of the cerebral seizure threshold when quinolones are given concurrently with other drugs that lower the seizure threshold (e.g. theophylline) or with fenbufen or similar non-steroidal anti-inflammatory drugs.
Antidiabetic agents: Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with quinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered.
Anticoagulant drug (warfarin and its derivatives): Coadministration with warfarin and its derivatives has been reported that the effect of warfarin was potentiated (hepatic metabolism of warfarin may be inhibited or free warfarin may be increased by competitive displacement from the protein binding site) and therefore prothrombin time prolonged.
Class IA antiarrhythmics and Class III antiarrhythmics: Levofloxacin should be used with caution in patients receiving drug known to cause QT prolonged, Class IA antiarrhythmics (e.g. quinidine sulfate and procainamide hydrochloride) Class III antiarrhythmics (e.g. amiodarone hydrochloride and sotalol hydrochloride) and Delamanid etc. QT prolongation may occur.
Regulatory classification:
1. Caution: Use only pursuant to the prescription or directions of a physician, etc.
2. Designated drugs
Nonproprietary name: Levofloxacin.
Abbreviation: LVFX.
Chemical name: (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido [1,2,3-de] [1,4] benzoxazine-6-carboxylic acid hemihydrate.
Molecular formula: C18H20FN3O4•½H2O.
Molecular weight: 370.38.
Melting point: 222 – 230°C (decomposition).
Description: Light yellowish-white to yellowish-white crystals or crystalline powder, odorless and bitter taste, freely soluble in glacial acetic acid, sparingly soluble in water and methanol, slightly soluble in ethanol and practically insoluble in ether. Light sensitive.
Pharmacology: Cravit is a broad-spectrum quinolone antibacterial agent containing levofloxacin, optically active (-)-S-form of racemate ofloxacin synthesized by Daiichi Sankyo Co., Ltd. Cravit shows broad and potent antibacterial activities against gram-positive bacteria such as Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes, Enterococcus faecalis, and gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae, Enterobacter cloacae, Moraxella catarrhalis, Legionella pneumophila, and other microorganisms such as Chlamydia pneumoniae and Mycoplasma pneumoniae. Cravit, which is transferred rapidly to each tissue in high concentrations without being accumulated there, is mostly excreted in the urine as unchanged form. Cravit shows clinical efficacy on respiratory tract infections, genitourinary tract infections and skin and skin structure infections.
Pharmacokinetics: Absorption and Serum concentration: Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained one to two hrs after oral dosing. The absolute bioavailability of a 500 mg tablet is approximately 99%, demonstrating complete oral absorption of levofloxacin. Levofloxacin pharmacokinetics is linear and predictable after single and multiple oral dosing regimens. The mean ± SD peak and trough plasma concentrations attained following multiple oral regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 μg/mL after the 500 mg doses and 8.6 ± 1.9 and 1.1 ± 0.4 μg/mL after 750 mg doses, respectively.
Distribution: The mean volume of distribution of levofloxacin generally ranges from 74 to 112 L after single and multiple 500 and 750 mg doses, indicating widespread distribution into body tissues. Levofloxacin reaches its peak levels in skin tissue and blister fluid of healthy subjects at approximately 3 hrs after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple oral administration of 750 mg and 500 mg levofloxacin, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2 to 5 folds higher than plasma concentrations and range from approximately 2.4 to 11.3 μg/g over 24 hour period after a single 500 mg oral dose.
In vitro, over a clinical relevant range (1-10 μg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24 to 38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Metabolism: Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered unchanged drug in urine within 48 hrs, whereas less than 4% of dose was recovered in feces in 72 hours. Less than 5% of an administered dose was recovered in urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Excretion: Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6 to 8 hours following single or multiple doses of levofloxacin given orally. The mean apparent total body clearance and renal clearance range from approximately 144 to 226 mL/min and 96 to 142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid result in approximately 24% and 35% reduction in the levofloxacin renal clearance, respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving levofloxacin.
Microbiology: Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. It is two folds stronger than that of ofloxacin. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair and recombination. Levofloxacin has in vitro activity against a wide range of gram-negative and gram-positive microorganisms. Levofloxacin is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from aminoglycosides, macrolides and β-lactam antibiotics including penicillins. Fluoroquinolones may, therefore, be active against bacteria resistant to these antimicrobials.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10-9 to 10-10). Although cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Levofloxacin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in Indications: Aerobic gram-positive microorganisms: Enterococcus faecalis, Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes.
Aerobic gram-negative microorganisms: Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Proteus mirabilis, Pseudomonas aeruginosa.
Other microorganisms: Chlamydia pneumoniae, Mycoplasma pneumoniae.
The following in vitro data are available, but their clinical significance is unknown.
Aerobic gram-positive microorganisms: Staphylococcus epidermidis, Streptococcus (Group C/F), Streptococcus (Group G), Streptococcus agalactiae, Streptococcus milleri, Viridans group streptococci.
Aerobic gram-negative microorganisms: Acinetobacter baumannii, Acinetobacter lwoffii, Bordetella pertussis, Citrobacter (diversus) koseri, Citrobacter freundii, Enterobacter aerogenes, Enterobacter sakazakii, Klebsiella oxytoca, Morganella morganii, Pantoea (Enterobacter) agglomerans, Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Pseudomonas fluorescens, Serratia marcescens.
Anaerobic gram-positive microorganisms: Clostridium perfringens.
FC tab (pale yellowish-white to reddish-white, oblong, biconvex) 250 mg x 5’s. 500 mg x 5’s, 10’s. 750 mg x 1 x 5’s.
Cravit tablets are indicated for the treatment of adults (≥ 16 years of age) with mild, moderate, and severe infections caused by susceptible strains of the designated microorganisms in the conditions listed as follows: Acute sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae or Moraxella catarrhalis.
Acute exacerbation of chronic bronchitis due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae or Moraxella catarrhalis.
Community-acquired pneumonia due to Staphylococcus aureus, Streptococcus pneumoniae (including penicillin-resistant strains), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydia pneumoniae, Legionella pneumophila or Mycoplasma pneumonia.
Nosocomial pneumonia due to methicillin-susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae or Streptococcus pneumoniae. Adjunctive therapy should be used as clinically indicated. Where Pseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an anti-pseudomonal β-lactam is recommended.
Uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma and wound infections due to Staphylococcus aureus and Streptococcus pyogenes.
Complicated skin and skin structure infections (mild to moderate) due to methicillin-susceptible Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes or Proteus mirabilis.
Urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa or Staphylococcus saprophyticus.
Chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis or Staphylococcus epidermidis.
Pyelonephritis (mild to moderate) caused by Escherichia coli.
Empirical treatment for community acquired pneumonia most likely caused by organisms susceptible to levofloxacin.
Third-line therapy for Helicobacter pylori infection: Levofloxacin-based Triple Therapy: Levofloxacin, in combination with other antimicrobial agent and proton-pump inhibitor as triple therapy, is indicated for the treatment of patients with gastric ulcer caused by Helicobacter pylori infection and doudenal ulcer disease.
Copyright MIMS
Empirical Treatment for Community-Acquired Pneumonia: 500 or 750 mg is administered once daily.
Patients with Normal Renal Function: See Table 1.

Patients with Impaired Renal Function: See Table 2.

When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.

The serum creatinine should represent a steady state of renal function.
Patients with Impaired Liver Function: No adjustment of dosage is required since levofloxacin is not metabolized to any relevant extent by liver and is mainly excreted by kidneys.
Elderly: No adjustment is required in the elderly, other than that imposed by consideration of renal function.
Copyright MIMS
According to toxicity studies in animals, the most important signs to be expected following acute overdosage of Cravit 500 mg or 750 mg are central nervous system symptoms such as confusion, dizziness, impairment of consciousness, and convulsive seizures, as well as gastrointestinal reactions such as nausea and mucosal erosions.
In the event of an acute overdosage, the stomach should be emptied. Antacid may be used for protection of gastric mucosa. No specific antidote exists. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Copyright MIMS
Levofloxacin is contraindicated in the following patients: Patients with a history of hypersensitivity to levofloxacin, ofloxacin or any excipients of this product.
Patients with epilepsy.
Patients with history of tendon disorder related to fluoroquinolones administration.
Children or adolescents below the age of 16 years.
Pregnant women or women suspected of being pregnant.
Breast-feeding women.
Copyright MIMS
Use during pregnancy: Levofloxacin must not be used in pregnant women or women suspected of being pregnant since the safety of the product in pregnant women has not been established (see CONTRAINDICATIONS).
Use during lactation: Since ofloxacin is known to be excreted in breast milk, nursing mothers should be guided to avoid the breast-feeding during treatment with levofloxacin (see CONTRAINDICATIONS).
Copyright MIMS
Empirical Treatment for Community-Acquired Pneumonia: 500 or 750 mg is administered once daily.
Patients with Normal Renal Function: See Table 1.

Patients with Impaired Renal Function: See Table 2.

When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.

The serum creatinine should represent a steady state of renal function.
Patients with Impaired Liver Function: No adjustment of dosage is required since levofloxacin is not metabolized to any relevant extent by liver and is mainly excreted by kidneys.
Elderly: No adjustment is required in the elderly, other than that imposed by consideration of renal function.
Copyright MIMS
Cravit Tab should be administered with caution in the following patients: Patients with severe renal impairment: Levofloxacin is excreted mainly by the kidneys and persistence of high serum level in patients with renal impairment has been reported.
Patients with known or suspected CNS disorder such as epilepsy or with a history of convulsive disease that may predispose to seizures or lower the seizure threshold, convulsion may occur.
Diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (especially, sulfonylureas or with insulin preparations).
Patients with a history of hypersensitivity to quinolone antibiotics.
Patients with serious heart diseases (e.g. arrhythmia and ischemic heart disease) patients with uncorrected electrolyte imbalance (e.g. hypokalemia, hypomagnesemia) and patients receiving class IA and III antiarrhythmic agents. QT prolongation may occur (see SERIOUS ADVERSE REACTION under ADVERSE REACTIONS and INTERACTIONS).
Patients with myasthenia gravis: Levofloxacin may cause exacerbation of myasthenia gravis symptoms.
Levofloxacin is more soluble than other quinolones, adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of highly concentrated urine.
Levofloxacin may inhibit the growth of Mycobacterium tuberculosis, and therefore, may give false-negative results in the bacteriological diagnosis of tuberculosis.
Some undesirable effects (see ADVERSE REACTIONS) may impair the patient’s ability to concentration and react, and therefore may constitute a risk in situation where these abilities are of special importance (e.g. driving a car or operating machinery).
Excessive exposure to sunlight should be avoided. However, phototoxicity has been observed very rare: incidence < 0.01%. Therapy should be discontinued if phototoxicity (e.g. a skin eruption) occurs.
Patients complicated with aortic aneurysm or aortic dissection, or patients who have a previous history, positive family history, or risk factors (Marfan syndrome, etc.) of aortic aneurysm or aortic dissection [The increased risk of aortic aneurysm and dissection after intake of fluoroquinolones have been reported in overseas epidemiologic studies (see SERIOUS ADVERSE REACTION under ADVERSE REACTIONS)].
Aortic aneurysm or aortic dissection may occur; therefore, patients should be carefully observed and instructed to seek medical attention immediately if they experience symptoms, e.g. in case of pain in the abdomen, chest, or back. Imaging assessment should be considered if necessary, for patients complicated with aortic aneurysm or aortic dissection, or patients who have a previous history, positive family history, or risk factors of aortic aneurysm or aortic dissection (see SERIOUS ADVERSE REACTION under ADVERSE REACTIONS).
Effect on ability to drive and use machine: Neurologic adverse effects such dizziness/vertigo and somnolence may occur. Therefore, patients should be instructed that such neurologic adverse effects may impair the patient’s ability to concentrate and react, and therefore may constitute a risk in situation where these abilities are of special importance (e.g. activities at high place, driving a car or operating machinery).
Use in the Elderly: Since renal function is generally depressed in geriatric patients and levofloxacin is excreted mainly by the kidneys, levofloxacin should be used with caution in this population (see PRECAUTION ASSOCIATED WITH DOSAGE AND ADMINISTRATION: GERIATRIC PATIENTS under DOSAGE & ADMINISTRATION).
Copyright MIMS
The following adverse reactions have been reported in clinical studies and post-marketing experience. The incidence identified as follows reflects exposure to 500 mg tablet of levofloxacin in total of 1,930 patients in pooled Phase 3 and Phase 4 clinical trials (i.e., 1,582 patients from Phase 3 clinical trials conducted in Japan (337 patients) and China (1,245 patients) and 348 patients from Phase 4 clinical trials) or 29,880 patients in a post marketing studies conducted in Japan. If the incidence category of an adverse reaction is different between each source (i.e., the incidence from the pooled clinical trials and the incidence from the post-marketing study), the higher frequency is represented.
The following CIOMS frequency rating is used: Very common: 10% ≤ incidence; Common: 1% ≤ incidence <10%; Uncommon: 0.1% ≤ incidence <1%; Rare: 0.01% ≤ incidence <0.1%; Very rare: incidence <0.01%.
*: see Serious Adverse Reaction in the following text. Each incidence is based on serious reactions.
Blood and lymphatic system disorders: Common: anemia.
Very rare: thrombocytopenia*.
Incidence unknown: pancytopenia*, agranulocytosis*, hemolytic anemia with hemoglobinuria*.
Immune system disorder: Incidence unknown: anaphylactoid reaction*.
Metabolism and nutrition disorder: Uncommon: anorexia.
Incidence unknown: hypoglycemia (hypoglycaemic coma may occur)*, hyperglycemia*.
Psychiatric disorders: Common: sleep loss.
Rare: hallucination.
Incidence Unknown: Psychiatric symptoms eg, confusion*, delirium*, depression*.
Nervous System Disorders: Common: dizziness/vertigo, headache.
Uncommon: somnolence, numbness, tremor, mental dullness, dysgeusia.
Rare: consciousness disturbed.
Very Rare: convulsion*, ageusia.
Incidence unknown: peripheral nerve disorder, extrapyramidal disorder, anosmia, parosmia.
Eye disorders: Rare: abnormal vision.
Ear and Labyrinth Disorders: Uncommon: tinnitus.
Incidence unknown: hearing losses.
Cardiac disorders: Uncommon: palpitations.
Incidence unknown: ventricular tachycardia (including Torsades de pointes)*, QT prolonged*, tachycardia.
Vascular disorders: Very rare: shock*. Incidence unknown: hypotension.
Respiratory, thoracic and mediastinal disorders: Uncommon: dry throat.
Incidence unknown: interstitial pneumonia*, eosinophilic pneumonia*.
Gastrointestinal disorders: Common: nausea, vomiting, diarrhea, abdominal discomfort.
Uncommon: abdominal pain, dyspepsia, abdominal distention, constipation.
Rare: stomatitis. Very rare: glossitis.
Incidence unknown: colitis with bloody stool, such as pseudomembranous colitis*.
Hepatobiliary Disorders: Uncommon: hepatic function abnormal (severe hepatic function disorder* may rarely occur.
Incidence unknown: hepatitis fulminant hepatitis*, jaundice*.
Skin and subcutaneous tissue disorders: Uncommon: pruritus, rash.
Rare: hyperhidrosis, urticaria.
Very rare: photosensitivity.
Incidence unknown: toxic epidermal necrolysis (TEN)*, oculomucocutaneous syndrome (Stevens-Johnson syndrome)*, hypersensitivity vasculitis*.
Musculoskeletal and connective tissue disorders: Uncommon: arthralgia, pain in extremity, back pain, weakness.
Rare: arthropathy, myalgia.
Incidence unknown: rhabdomyolysis*, tendon disorders such as Achilles tendonitis or tendon rupture*, exacerbation of myasthenia gravis*, muscle rupture.
Renal and urinary disorders: Uncommon: hematuria, urinary distention.
Rare: pollakiuria, oliguria, acute renal failure*.
Incidence unknown: interstitial nephritis*, anuria, dysuria.
General disorders and administration site conditions: Uncommon: thirst, chest discomfort, malaise, feeling hot, edema.
Very Rare: pyrexia.
Incidence unknown: chest pain.
Investigations: Common: AST increased, ALT increased, LDH increased, white blood cell count decreased, eosinophil count increased.
Uncommon: creatinine increased, urinary protein positive, alkaline phosphatase increased, γ-GTP increased, blood bilirubin increased, lymphocyte count decreased, neutrophil count decreased, CPK increased, glucose urine present, blood lucose decreased, platelet count decreased.
Rare: BUN increased, urine output decreased.
Very Rare: blood glucose increased.
*See Serious Adverse Reaction in the following text.
Serious Adverse Reaction: The following serious adverse reactions have been reported in patients receiving therapy with levofloxacin. If the following reactions are suspected, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measure should be taken: Shock or anaphylactoid reaction (initial symptoms: erythema, rigor, dyspnea, etc.).
Toxic epidermal necrolysis (TEN) or oculomucocutaneous syndrome (Stevens-Johnson syndrome).
Convulsion.
QT prolonged and ventricular tachycardia (including Torsades de pointes): During post-marketing surveillance, prolonged QT which may sometimes lead to the occurrence of ventricular tachycardia including torsades de pointes have been reported spontaneously in patients taking levofloxacin. The risk of the events may be increased in patients with serious heart diseases (e.g. arrhythmia and ischemic heart disease), patients with uncorrected hypokalemia, patients receiving Class IA (quinidine sulfate, procainamide hydrochloride) and Class III (amiodarone hydrochloride, sotalol hydrochloride) antiarrhythmic agents and in geriatric patients.
Acute renal failure or interstitial nephritis.
Hepatitis fulminant, hepatic function disorder or jaundice (initial symptoms: nausea, vomiting, anorexia, malaise, pruritus, etc.).
Pancytopenia, agranulocytosis (initial symptoms: pyrexia, pharynx pain, malaise, etc.), hemolytic anemia with hemoglobinuria or thrombocytopenia.
Interstitial pneumonia or eosinophilic pneumonia accompanied with pyrexia, cough, dyspnea, abnormal chest X-ray, or eosinophilia, etc.
Serious colitis with bloody stool, such as pseudomembranous colitis: If such symptoms as abdominal pain and frequent diarrhea are noted, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measures taken.
Rhabdomyolysis characterized by myalgia, weakness, elevated CK (CPK) and increased myoglobin in plasma and urine, etc., and accompanied with acute exacerbation of renal function.
Dysglycemia: During post-marketing surveillance, hypoglycemia and hyperglycemia have been reported in patients taking levofloxacin. Serious symptoms such as hypoglycemic coma have been reported in patients receiving levofloxacin. Hypoglycemia may be prone to develop in patients with diabetes mellitus (especially, those receiving sulfonylureas or insulin preparations), patients with impaired renal function and geriatric patients.
Tendon disorders such as Achilles tendonitis or tendon rupture: If symptoms such as pain and edema in the peritendinous region are observed, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measures taken. The risk of tendonitis and tendon rupture is increased in those over age 60, in those on concomitant corticosteroid therapy, and transplant recipients.
Psychiatric symptoms such as confusion, delirium and depression.
Hypersensitivity vasculitis: If symptoms such as pyrexia, abdominal pain, arthralgia, purpura or maculopapules, and skin biopsy evidence of leukocytoclastic vasculitis are observed, treatment with levofloxacin should be discontinued immediately and appropriate therapeutic measures taken.
Exacerbation of myasthenia gravis.
Patients received Levofloxacin dose of 750 mg may develop some adverse reaction such as dizziness, headache, nausea or vomiting more than Levofloxacin dose of 500 mg.
Aortic aneurysm, aortic dissection (incidence unknown): Aortic aneurysm or aortic dissection may occur. If any abnormalities are observed, appropriate medical treatment should be taken (see Precautions).
Copyright MIMS
Antacid, Sucralfate, Metal Cations, Multivitamins: While the chelation by divalent cations is less marked than with other quinolones, concurrent administration of levofloxacin with antacids containing magnesium or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least two hours before or two hours after levofloxacin administration.
Theophylline, Fenbufen or similar non-steroidal anti-inflammatory drugs (phenylacetic acid/propionic acid derivatives): No pharmacokinetic interactions of levofloxacin were found with theophylline in a clinical study. However there are indications of a pronounced lowering of the cerebral seizure threshold when quinolones are given concurrently with other drugs that lower the seizure threshold (e.g. theophylline) or with fenbufen or similar non-steroidal anti-inflammatory drugs.
Antidiabetic agents: Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with quinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered.
Anticoagulant drug (warfarin and its derivatives): Coadministration with warfarin and its derivatives has been reported that the effect of warfarin was potentiated (hepatic metabolism of warfarin may be inhibited or free warfarin may be increased by competitive displacement from the protein binding site) and therefore prothrombin time prolonged.
Class IA antiarrhythmics and Class III antiarrhythmics: Levofloxacin should be used with caution in patients receiving drug known to cause QT prolonged, Class IA antiarrhythmics (e.g. quinidine sulfate and procainamide hydrochloride) Class III antiarrhythmics (e.g. amiodarone hydrochloride and sotalol hydrochloride) and Delamanid etc. QT prolongation may occur.
Copyright MIMS
Regulatory classification:
1. Caution: Use only pursuant to the prescription or directions of a physician, etc.
2. Designated drugs
Copyright MIMS
Copyright MIMS
Nonproprietary name: Levofloxacin.
Abbreviation: LVFX.
Chemical name: (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido [1,2,3-de] [1,4] benzoxazine-6-carboxylic acid hemihydrate.
Molecular formula: C18H20FN3O4•½H2O.
Molecular weight: 370.38.
Melting point: 222 – 230°C (decomposition).
Description: Light yellowish-white to yellowish-white crystals or crystalline powder, odorless and bitter taste, freely soluble in glacial acetic acid, sparingly soluble in water and methanol, slightly soluble in ethanol and practically insoluble in ether. Light sensitive.
Copyright MIMS
Pharmacology: Cravit is a broad-spectrum quinolone antibacterial agent containing levofloxacin, optically active (-)-S-form of racemate ofloxacin synthesized by Daiichi Sankyo Co., Ltd. Cravit shows broad and potent antibacterial activities against gram-positive bacteria such as Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes, Enterococcus faecalis, and gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae, Enterobacter cloacae, Moraxella catarrhalis, Legionella pneumophila, and other microorganisms such as Chlamydia pneumoniae and Mycoplasma pneumoniae. Cravit, which is transferred rapidly to each tissue in high concentrations without being accumulated there, is mostly excreted in the urine as unchanged form. Cravit shows clinical efficacy on respiratory tract infections, genitourinary tract infections and skin and skin structure infections.
Pharmacokinetics: Absorption and Serum concentration: Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained one to two hrs after oral dosing. The absolute bioavailability of a 500 mg tablet is approximately 99%, demonstrating complete oral absorption of levofloxacin. Levofloxacin pharmacokinetics is linear and predictable after single and multiple oral dosing regimens. The mean ± SD peak and trough plasma concentrations attained following multiple oral regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 μg/mL after the 500 mg doses and 8.6 ± 1.9 and 1.1 ± 0.4 μg/mL after 750 mg doses, respectively.
Distribution: The mean volume of distribution of levofloxacin generally ranges from 74 to 112 L after single and multiple 500 and 750 mg doses, indicating widespread distribution into body tissues. Levofloxacin reaches its peak levels in skin tissue and blister fluid of healthy subjects at approximately 3 hrs after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple oral administration of 750 mg and 500 mg levofloxacin, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2 to 5 folds higher than plasma concentrations and range from approximately 2.4 to 11.3 μg/g over 24 hour period after a single 500 mg oral dose.
In vitro, over a clinical relevant range (1-10 μg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24 to 38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Metabolism: Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered unchanged drug in urine within 48 hrs, whereas less than 4% of dose was recovered in feces in 72 hours. Less than 5% of an administered dose was recovered in urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Excretion: Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6 to 8 hours following single or multiple doses of levofloxacin given orally. The mean apparent total body clearance and renal clearance range from approximately 144 to 226 mL/min and 96 to 142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid result in approximately 24% and 35% reduction in the levofloxacin renal clearance, respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving levofloxacin.
Microbiology: Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. It is two folds stronger than that of ofloxacin. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair and recombination. Levofloxacin has in vitro activity against a wide range of gram-negative and gram-positive microorganisms. Levofloxacin is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from aminoglycosides, macrolides and β-lactam antibiotics including penicillins. Fluoroquinolones may, therefore, be active against bacteria resistant to these antimicrobials.
Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10-9 to 10-10). Although cross-resistance has been observed between levofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to levofloxacin.
Levofloxacin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in Indications: Aerobic gram-positive microorganisms: Enterococcus faecalis, Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes.
Aerobic gram-negative microorganisms: Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Proteus mirabilis, Pseudomonas aeruginosa.
Other microorganisms: Chlamydia pneumoniae, Mycoplasma pneumoniae.
The following in vitro data are available, but their clinical significance is unknown.
Aerobic gram-positive microorganisms: Staphylococcus epidermidis, Streptococcus (Group C/F), Streptococcus (Group G), Streptococcus agalactiae, Streptococcus milleri, Viridans group streptococci.
Aerobic gram-negative microorganisms: Acinetobacter baumannii, Acinetobacter lwoffii, Bordetella pertussis, Citrobacter (diversus) koseri, Citrobacter freundii, Enterobacter aerogenes, Enterobacter sakazakii, Klebsiella oxytoca, Morganella morganii, Pantoea (Enterobacter) agglomerans, Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Pseudomonas fluorescens, Serratia marcescens.
Anaerobic gram-positive microorganisms: Clostridium perfringens.
Copyright MIMS
Copyright MIMS
FC tab (pale yellowish-white to reddish-white, oblong, biconvex) 250 mg x 5’s. 500 mg x 5’s, 10’s. 750 mg x 1 x 5’s.
Copyright MIMS









