CRAVIT

The product information provided in this page is intended only for the selected country.
Cravit contains levofloxacin as an active ingredient, and is used to treat a variety of bacterial infections. This medication belongs to a class of drugs known as quinolone antibiotics and works by inhibiting the growth of bacteria. It is usually used to treat a range of infections including skin, respiratory, urinary tract, gynaecologic and otologic infections.
Treatment of adults (more than or equal to 18 years) with mild, moderate and severe infections caused by susceptible strains of the designated microorganisms In the conditions listed as follows. Cravit injection is indicated when i.v. administration offers a route of administration advantageous to the patient (e.g., patient cannot tolerate an oral dosage form). (See Dosage & Administration for specific recommendations.)
Acute maxillary sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis.
Acute bacterial exacerbation of chronic bronchitis due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Community-acquired pneumonia due to Staphylococcus aureus, Streptococcus pneumoniae, Legionella pneumophila or Mycoplasma pneumoniae.
Uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections due to Staphylococcus aureus, or Streptococcus pyogenes.
Complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa.
Acute pyelonephritis (mild to moderate) caused by Escherichia coli.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin. Therapy with levofloxacin may be initiated before results of these tests are known, once results become available, appropriate therapy should be selected.
As with other drugs in the class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with levofloxacin. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.
Cravit FC Tab: For adults, 100 mg (1 tab) of levofloxacin is usually administered orally 2-3 times a day. The dosage may be adjusted according to the kind of infection and symptoms. In a severe case or insufficient efficacy, 200 mg (2 tab) of levofloxacin is administered 3 times a day.
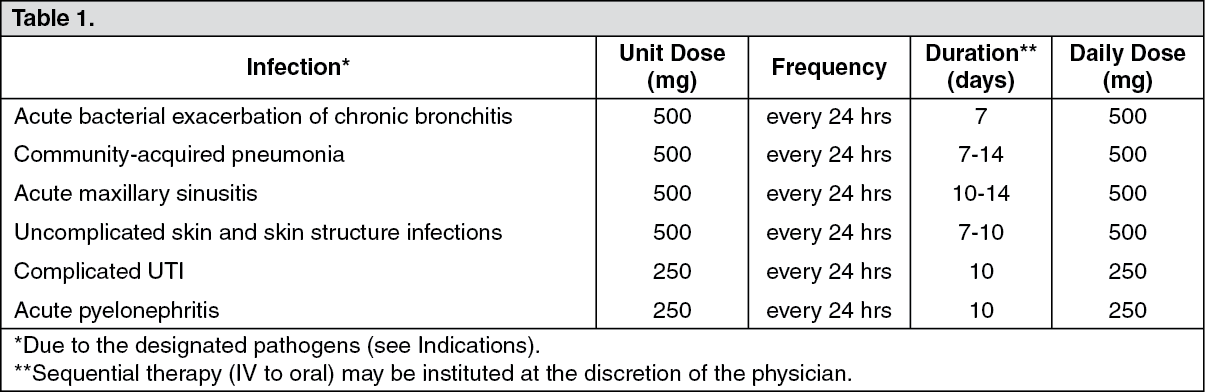
Usual Dose: 250 or 500 mg administered by slow infusion over 60 min every 24 hrs or 750 mg administered orally or by slow infusion over 90 min every 24 hrs, as indicated by infection and described in the following dosing chart. These recommendations apply to patients with normal renal function (ie, creatinine clearance >80 mL/min). For patients with altered renal function, see Table 2. Oral doses should be administered at least 2 hrs before or 2 hrs after antacids containing magnesium, aluminum, as well as sucralfate, metal cations eg, iron and multivitamin preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution.
Careful administration should be given to patients with severe renal disorders, history of convulsive diseases eg, epilepsy (convulsions may possibly occur), history of hypersensitivity to quinolone antibacterial agents and the elderly.
Cravit IV Infusion: Cravit injection should only be administered by IV infusion. It is not for IM, intrathecal, intraperitoneal or SC administration.
Note: Rapid or bolus IV infusion must be avoided. Levofloxacin injection should be infused IV slowly over a period of not less than 60 or 90 min, depending on the dosage (see Precautions).
Patients with Normal Renal Function: See Table 1.
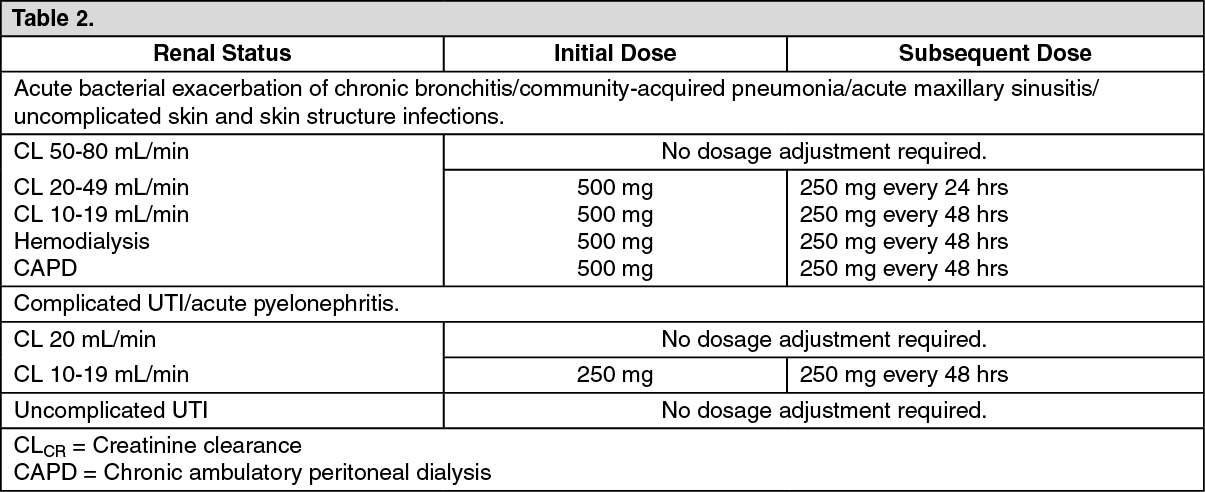
Patients with Impaired Renal Function: See Table 2.
When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.
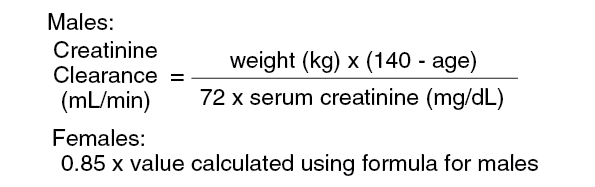
The serum creatinine should represent a steady state of renal function.
Levofloxacin exhibits a low potential for acute toxicity. Mice, rats, dogs and monkeys exhibited the following clinical signs after receiving a single high dose of levofloxacin: Ataxia, ptosis, decreased locomotor activity, dyspnea, prostration, tremors and convulsions. Doses in excess of 1500 mg/kg orally and 250 mg/kg i.v. produced significant mortality in rodents. In the event of an acute overdosage, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Persons with a history of hypersensitivity to levofloxacin, quinolone antimicrobial agents, or any other components of Cravit.
Fluoroquinolones, including Levofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
Patients experiencing pain, swelling, inflammation of a tendon or tendon rupture should be advised to stop taking Levofloxacin and to contact their health care professional promptly about changing their antimicrobial therapy. Patients should also avoid exercise and using the affected area at the first sign of tendon pain, swelling, or inflammation. Tendon rupture can occur during or after completion of therapy; cases occuring up to several months after completion of therapy have been reported.
Exacerbation or myasthenia gravis: Fluoroquinolones, including levofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in person with myasthenia gravis.
Post marketing serious adverse events, including deaths and requirement for ventilator support have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid levofloxacin in patients with known history of myasthenia gravis.
The safety and efficacy of levofloxacin in children, adolescents (<18 years), pregnant and nursing women have not been established (see Use in pregnancy, Use in lactation under Use in Pregnancy & Lactation and Use in children under Precautions).
In immature rats and dogs, the oral and i.v. administration of levofloxacin increased the incidence and severity of osteochondrosis. Other fluoroquinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species (see Pharmacology under Actions).
Convulsions and toxic psychoses have been reported in patients receiving quinolones, including levofloxacin. Quinolones may also cause increased intracranial pressure and central nervous system stimulation which may lead to tremors, restlessness, anxiety, lightheadedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia and rarely, suicidal thoughts or acts. These reactions may occur following the 1st dose. If these reactions occur in patients receiving levofloxacin, the drug should be discontinued and appropriate measures instituted. As with other quinolones, levofloxacin should be used with caution in patients with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction) (see Information for Patients under Precautions, Interactions and Adverse Reactions).
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones including levofloxacin. These reactions often occur following the 1st dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/ swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching and other serious skin reactions. Levofloxacin should be discontinued immediately at the first appearance of a skin rash or any other signs of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, i.v. fluids, antihistamines, corticosteroids, pressor amines and airway management, as clinically indicated (see Precautions and Adverse Reactions).
Serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones including levofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following: fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome); vasculitis; arthralgia; myalgia; serum sickness; allergic pneumonitis; interstitial nephritis; acute renal insufficiency or failure; hepatitis; jaundice; acute hepatic necrosis or failure; anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities. Cravit should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity and supportive measures instituted (see Information for Patients under Precautions and Adverse Reactions).
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including levofloxacin, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug clinically effective against C. difficile colitis (see Adverse Reactions).
Use in pregnancy: There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies potential risk to the fetus (see Warnings).
Use in lactation: Levofloxacin has not been measured in human milk. Based upon data from ofloxacin, it can be presumed that levofloxacin will be excreted in human milk. Because of the potential for serious adverse reactions from levofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
Cravit FC Tab: For adults, 100 mg (1 tab) of levofloxacin is usually administered orally 2-3 times a day. The dosage may be adjusted according to the kind of infection and symptoms. In a severe case or insufficient efficacy, 200 mg (2 tab) of levofloxacin is administered 3 times a day.
Usual Dose: 250 or 500 mg administered by slow infusion over 60 min every 24 hrs or 750 mg administered orally or by slow infusion over 90 min every 24 hrs, as indicated by infection and described in the following dosing chart. These recommendations apply to patients with normal renal function (ie, creatinine clearance >80 mL/min). For patients with altered renal function, see Table 2. Oral doses should be administered at least 2 hrs before or 2 hrs after antacids containing magnesium, aluminum, as well as sucralfate, metal cations eg, iron and multivitamin preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution.
Careful administration should be given to patients with severe renal disorders, history of convulsive diseases eg, epilepsy (convulsions may possibly occur), history of hypersensitivity to quinolone antibacterial agents and the elderly.
Cravit IV Infusion: Cravit injection should only be administered by IV infusion. It is not for IM, intrathecal, intraperitoneal or SC administration.
Note: Rapid or bolus IV infusion must be avoided. Levofloxacin injection should be infused IV slowly over a period of not less than 60 or 90 min, depending on the dosage (see Precautions).
Patients with Normal Renal Function: See Table 1.

Patients with Impaired Renal Function: See Table 2.

When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.
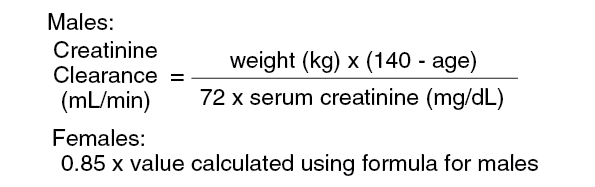
The serum creatinine should represent a steady state of renal function.
General: Because a rapid or bolus i.v. injection may result in hypotension, levofloxacin injection should only be administered by slow i.v, infusion over a period of 60 or 90 min depending on the dosage (see Dosage & Administration).
Although levofloxacin is more soluble than other quinolones, adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of a highly concentrated urine.
Administer levofloxacin with caution in the presence of renal insufficiency. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced. In patients with impaired renal function (creatinine clearance <50 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance. (See Pharmacology: Pharmacokinetics under Actions and Dosage & Administration.)
Moderate to severe phototoxicity reactions have been observed in patients exposed to direct sunlight while receiving drugs in this class. Excessive exposure to sunlight should be avoided. However, in clinical trials with levofloxacin, phototoxicity has been observed in <0.1% of patients. Therapy should be discontinued if phototoxicity (e.g., a skin eruption) occurs.
As with other quinolones, levofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction). (See Warnings and Interactions.)
As with other quinolones, disturbances of blood glucose, including symptomatic hyper- and hypoglycemia, have been reported, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide/glibenclamide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. If a hypoglycemic reaction occurs in a patient being treated with levofloxacin, levofloxacin should be discontinued immediately and appropriate therapy should be initiated immediately. (See Interactions and Adverse Reactions.)
Some quinolones, including levofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram (see Pharmacology: Electrocardiogram under Actions) and infrequent cases of arrhythmia. During post-marketing surveillance, very rare cases of torsades de pointes have been reported in patients taking levofloxacin. These reports generally involved patients with concurrent medical conditions or concomitant medications that may have been contributory. The risk of arrhythmias may be reduced by avoiding concurrent use with other drugs that prolong the QT interval including class Ia or class III antiarrhythmic agents; In addition, use of levofloxacin in the presence of risk factors for torsades de pointes such as hypokalemia, significant bradycardia, and cardiomyopathy should be avoided.
As with any potent antimicrobial drug, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during therapy. (See Warnings and Adverse Reactions.)
Information for Patients: Patients should be advised: to drink fluids liberally; that antacids containing magnesium or aluminum, as well as sucralfate, metal cations, e.g. iron, and multivitamin preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution should be taken at least 2 hrs before or 2 hrs after oral levofloxacin administration (see Interactions); that oral levofloxacin can be taken without regard to meals; to discontinue treatment and inform the physician if the patient experiences pain, inflammation or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded; that levofloxacin may be associated with hypersensitivity reactions, even following the 1st dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (e.g., swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction (see Warnings and Adverse Reactions); to avoid excessive sunlight or artificial ultraviolet light while receiving levofloxacin and to discontinue therapy if phototoxicity (i.e., skin eruption) occurs; that if diabetic and being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, levofloxacin should be discontinued and consult a physician (see Interactions); that concurrent administration of warfarin and levofloxacin has been associated with increases of the International Normalized Ratio (INR) or prothrombin time and clinical episodes of bleeding. Patients should notify their physician if they are taking warfarin; that convulsions have been reported in patients taking quinolones, including levofloxacin and to notify their physician before taking Cravit if there is a history of this condition.
Effects on the Ability to Drive or Operate Machinery: Patients should be advised that levofloxacin may cause neurologic adverse effects (e.g., dizziness, lightheadedness) and they should know how to react to levofloxacin before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination (see Warnings and Adverse Reactions).
Carcinogenicity, Mutagenicity & Impairment of Fertility: In a lifetime bioassay in rats, levofloxacin exhibited no carcinogenic potential following daily dietary administration for 2 years; the highest dose (100 mg/kg/day) was 1.4 times the highest recommended human dose (750 mg) based upon relative body surface area.
Levofloxacin was not mutagenic in the following assays: Ames bacterial mutation assay (S. typhimurium and E. coli), CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and sister chromatid exchange (CHL/IU cell line) assays.
Levofloxacin caused no impairment of fertility or reproductive performance in rats at oral doses as high as 360 mg/kg/day, corresponding to 4.2 times the highest recommended human dose based upon relative body surface area and i.v. doses as high as 100 mg/kg/day, corresponding to 1.2 times the highest recommended human dose based upon relative body surface area.
Teratogenicity: Pregnancy Category C: Levofloxacin was not teratogenic in rats at oral doses as high as 810 mg/kg/day, which corresponds to 9.4 times the highest recommended human dose based upon relative body surface area or at i.v. doses as high as 160 mg/kg/day, corresponding to 1.9 times the highest recommended human dose based upon relative body surface area. The oral dose of 810 mg/kg/day to rats caused decreased fetal body weight and increased fetal mortality. No teratogenicity was observed when rabbits were dosed orally as high as 50 mg/kg/day, which corresponds to 1.1 times the highest recommended human dose based upon relative body surface area or when dosed intravenously as high as 25 mg/kg/day, corresponding to 0.5 times the highest recommended human dose based upon relative body surface area.
Use in pregnancy: There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies potential risk to the fetus (see Warnings).
Use in lactation: Levofloxacin has not been measured in human milk. Based upon data from ofloxacin, it can be presumed that levofloxacin will be excreted in human milk. Because of the potential for serious adverse reactions from levofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
Use in children: Safety and effectiveness in pediatric patients and adolescents <18 years have not been established. Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species (see Warnings.)
Use in the elderly: In phase 3 clinical trials, 1190 levofloxacin-treated patients (25%) were more than or equal to 65 years. Of these, 675 patients (14%) were between 65 and 74 years and 515 patients (11%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However, since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to levofloxacin may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
The incidence of drug-related adverse reactions in patients during Phase 3 clinical trials conducted in North America was 6.3%. Among patients receiving levofloxacin therapy, 3.9% discontinued levofloxacin therapy due to adverse experiences. The overall incidence, type and distribution of adverse events were similar in patients receiving levofloxacin doses of 750 mg once daily compared to patients receiving doses from 250 mg once daily to 500 mg twice daily.
In clinical trials, the following events were considered likely to be drug-related in patients receiving levofloxacin: nausea 1.3%, diarrhea 1%, vaginitis 0.7%, insomnia 0.5%, abdominal pain 0.4%, flatulence 0.4%, pruritus 0.4%, dizziness 0.3%, dyspepsia 0.3%, rash 0.3%, genital moniliasis 0.2%, taste perversion 0.2%, vomiting 0.2%, constipation 0.1%, fungal infection 0.1%, genital pruritis 0.1%, headache 0.1%, moniliasis 0.1%, nervousness 0.1%, erythematous rash 0.1%, urticaria 0.1%.
In clinical trials, the following events occurred in >3%, regardless of drug relationship: Nausea 7.2%, headache 6.4%, diarrhea 5.6%, insomnia 4.6%, injection site reaction 3.5% and constipation 3.2%.
In clinical trials, the following events occurred in 1-3% of patients, regardless of drug relationship: Dizziness 2.7%, abdominal pain 2.5%, dyspepsia 2.4%, vomiting 2.3%, vaginitis 1.8%, injection site pain 1.7%, flatulence 1.5%, pain 1.4%, pruritus 1.3%, sinusitis 1.3%, chest pain 1.2%, fatigue 1.2%, rash 1.2%, back pain 1.1%, injection site inflammation 1.1%, rhinitis 1% and taste perversion 1%.
In clinical trials, the following events, of potential medical importance, occurred at a rate of <1%, regardless of drug relationship: Autonomic Nervous System Disorders: Postural hypotension.
Body as a Whole-General Disorders: Asthenia, edema, fever, malaise, rigors, substernal chest pain and syncope.
General Cardiovascular Disorders: Cardiac failure, circulatory failure, hypertension and hypotension.
Central and Peripheral Nervous System Disorders: Abnormal coordination, coma, convulsions (seizures), hyperkinesia, hypertonia, hypoesthesia, involuntary muscle contractions, paresthesia, paralysis, speech disorder, stupor, tremor and vertigo.
Gastrointestinal System Disorders: Dry mouth, dysphagia, gastroenteritis, G.I. hemorrhage, pancreatitis, pseudomembranous colitis and tongue edema.
Hearing and Vestibular Disorders: Ear disorder (not otherwise specified) and tinnitus.
Heart Rate and Rhythm Disorders: Arrhythmia, atrial fibrillation, bradycardia, cardiac arrest, heart block, palpitation, supraventricular tachycardia, tachycardia and ventricular fibrillation.
Liver and Biliary System Disorders: Abnormal hepatic function, cholelithiasis, hepatic coma and jaundice.
Metabolic and Nutritional Disorders: Aggravated diabetes mellitus, dehydration, hyperglycemia, hyperkalemia, hypoglycemia, hypokalemia, increased LDH and decreased weight.
Musculoskeletal System Disorders: Arthralgia, arthritis, arthrosis, muscle weakness, myalgia, osteomyelitis, rhabdomyolysis, synovitis and tendinitis.
Myo-, Endo-, Pericardial and Valve Disorders: Angina pectoris, coronary thrombosis and myocardial infarction.
Neoplasms: Carcinoma.
Other Special Senses Disorders: Parosmia.
Platelet, Bleeding and Clotting Disorders: Abnormal platelets, embolism (blood clot), epistaxis, purpura and thrombocytopenia.
Psychiatric Disorders: Abnormal dreaming, aggressive reaction, agitation, anorexia, anxiety, confusion, delirium, depression, emotional lability, hallucination, impaired concentration, impotence, manic reaction, mental deficiency, nervousness, paranoia, sleep disorder, somnolence and withdrawal syndrome.
Red Blood Cell Disorders: Anemia.
Reproductive Disorders: Ejaculation failure.
Resistance Mechanism Disorders: Fungal infection and genital moniliasis.
Respiratory System Disorders: ARDS, asthma, coughing, dyspnea, haemoptysis, hypoxia, pleural effusion and respiratory insufficiency.
Skin and Appendages Disorders: Erythema nodosum, genital pruritus, increased sweating, skin disorder, skin exfoliation, skin ulceration and urticaria.
Urinary System Disorders: Abnormal renal function, acute renal failure, face edema and haematuria.
Vascular (Extracardiac) Disorders: Cerebrovascular disorder and phlebitis.
Vision Disorders: Abnormal vision, conjunctivitis and diplopia.
White Cell and RES Disorders: Granulocytopenia, leukocytosis, leukopenia, lymphadenopathy, abnormal WBC (not otherwise specified).
In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with other quinolones. The relationship of the drugs to these events is not presently established.
Crystalluria and cylindruria have been reported with other quinolones.
The following laboratory abnormalities appeared in 2.2% of patients receiving levofloxacin. It is not known whether these abnormalities were caused by the drug or the underlying condition being treated.
Blood Chemistry: Decreased glucose.
Hematology: Decreased lymphocytes.
Post-Marketing Adverse Reactions: Additional adverse events reported from worldwide post-marketing experience with levofloxacin include: Allergic pneumonitis, anaphylactic shock, anaphylactoid reaction, dysphonia, abnormal EEG, encephalopathy, eosinophilia, erythema multiforme, hemolytic anemia, multisystem organ failure, increased International Normalized Ratio (INR)/prothrombin time, Stevens-Johnson syndrome, tendon rupture, torsade de pointes and vasodilation, Exacerbation of myasthenia gravis.
Antacids, Sucralfate, Metal Cations, Multivitamins: Tablet: While the chelation by divalent cations is less marked than with other quinolones, concurrent administration of Cravit tablets with antacids containing magnesium or aluminum, as well as sucralfate, metal cations, such as iron, and multivitamin preparations with zinc, may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. Tablets with antacids containing magnesium, aluminum, as well as sucralfate, metal cations, such as iron and multivitamins preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution may substantially interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least 2 hrs before or 2 hrs after levofloxacin administration.
Injection: There are no data concerning an interaction of i.v. quinolones with oral antacids, sucralfate, multivitamins, didanosine (Videx), or metal cations. However, no quinolone should be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same i.v. line. (See Dosage & Administration.)
Theophylline: No significant effect of levofloxacin on the plasma concentrations, AUC and other disposition parameters for theophylline was detected in a clinical study involving 14 healthy volunteers. Similarly, no apparent effect of theophylline on levofloxacin absorption and disposition was observed. However, concomitant administration of other quinolones with theophylline has resulted in prolonged elimination half-life, elevated serum theophylline levels, and a subsequent increase in the risk of theophylline-related adverse reactions in the patient population. Therefore, theophylline levels should be closely monitored and appropriate dosage adjustments made when levofloxacin is co-administered. Adverse reactions, including seizures, may occur with or without an elevation in serum theophylline levels. (See Warnings and General under Precautions.)
Warfarin: No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for R- and S-warfarin was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of warfarin on levofloxacin absorption and disposition was observed. There have been reports during the post-marketing experience in patients that levofloxacin enhances the effects of warfarin. Elevations of the prothrombin time in the setting of concurrent warfarin and levofloxacin use have been associated with episodes of bleeding. Prothrombin time, International Normalized Ratio (INR) or other suitable anticoagulation tests should be closely monitored if levofloxacin is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding.
Cyclosporine: No significant effect of levofloxacin on the peak plasma concentrations, AUC and other disposition parameters for cyclosporine was detected in a clinical study involving healthy volunteers. However, elevated serum levels of cyclosporine have been reported in the patient population when co-administered with some other quinolones. Levofloxacin Cmax and Ke were slightly lower while Tmax and t½ were slightly longer in the presence of cyclosporine than those observed in other studies without concomitant medication. The differences, however, are not considered to be clinically significant. Therefore, no dosage adjustment is required for levofloxacin or cyclosporine when administered concomitantly.
Digoxin: No significant effect of levofloxacin on the peak plasma concentrations, AUC and other disposition parameters for digoxin was detected in a clinical study involving healthy volunteers. Levofloxacin absorption and disposition kinetics were similar in the presence or absence of digoxin. Therefore, no dosage adjustment for levofloxacin or digoxin is required when administered concomitantly.
Probenecid and Cimetidine: No significant effect of probenecid or cimetidine on the rate and extent of levofloxacin absorption was observed in a clinical study involving healthy volunteers. The AUC and t½ of levofloxacin were 27-38% and 30% higher, respectively, while CL/F and CLR were 21-35% lower during concomitant treatment with probenecid or cimetidine compared to levofloxacin alone. Although these differences were statistically significant, the changes were not high enough to warrant dosage adjustment for levofloxacin when probenecid or cimetidine is co-administered.
Non-steroidal anti-inflammatory drugs: The concomitant administration of a nonsteroidal anti-inflammatory drug with a quinolone, including levofloxacin, may increase the risk of CNS stimulation and convulsive seizures. (See Warnings and General under Precautions.)
Antidiabetic Agents: Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with quinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered.
Inspect visually for particulate matter prior to administration. Samples containing visible particles should be discarded. Once the infusion bottle has been opened (rubber stopper perforated) the solution should be used immediately (within 3 hours) in order to prevent any bacterial contamination.
Compatibilities/Incompatibilities: Since Cravit i.v. solution is ready for use, it may be given alone or with one of the following solutions: 0.9% Sodium chloride solution, 5% dextrose injection, 2.5% dextrose in Ringer solution and combination solutions for parenteral nutrition (amino acids, carbohydrates, electrolytes).
Cravit i.v. solution for infusion should not be mixed with certain other solutions (e.g., sodium hydrogen carbonate) or with heparin.
Store below 30°C in well-closed containers. Protect from light.
Use within 3 days after removal from the carton.
Each film-coated tablet of CRAVIT 250mg contains 250mg of levofloxacin as active ingredient corresponding to 256.23mg of levofloxacin hemihydrate and each film-coated tablet of CRAVIT 500mg contains 500mg of levofloxacin as active ingredient corresponding to 512.46mg of levofloxacin hemihydrate.
Cravit i.v. 5mg/ml is a sterile clear greenish-yellow solution for intravenous administration. Each 50ml bottle contains 250mg of levofloxacin as active substance. Each 100ml bottle contains 500mg of levofloxacin as active substance.
The solution also contains the following ingredients: sodium chloride; sodium hydroxide; hydrochloric acid (qs: pH 4.8) and water for injection (Na+ concentration: 154mmol/L).
CRAVIT (levofloxacin) Tablets/Injection are synthetic broad spectrum antibacterial agents for oral and intravenous administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The chemical name is (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-5-carboxylic acid hemihydrate.
Its empirical formula of C18H20FN3O4·½H2O and its molecular weight is 370.38. Levofloxacin is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine. The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin is essentially constant (approximately 100 mg/mL). Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9. Levofloxacin has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Al+3>Cu+2>Zn+2>Mg+2>Ca+2.
Synthetic broad-spectrum antibacterial agent for oral and i.v. administration.
Pharmacology: The main mechanism of action of levofloxacin is inhibition of DNA gyrase. It is 2-fold stronger than that of ofloxacin. There is not much difference between MIC and MBC. The activity of levofloxacin is bactericidal. In the observation of bacterial morphology, bacteriolysis can be seen in the concentration around MIC.
Cravit shows clinical efficacy on respiratory tract infections, genitourinary tract infections, biliary tract infections, intestinal tract infections and other various infections in the surgical, gynecological, dermatological, otorhinolaryngological, ophthalmological, and dental and oral surgery fields.
Pharmacokinetics: Absorption: Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained 1-2 hrs after oral dosing. The absolute bioavailability of a 500- and 750-mg tablet of levofloxacin are both approximately 99%, demonstrating complete oral absorption of levofloxacin. Following a single i.v. dose of levofloxacin to healthy volunteers, the mean ±SD peak plasma concentration attained was 6.2 ± 1.0 μg/mL after a 500-mg dose infused over 60 min and 11.5 ± 4.0 μg/mL after a 750-mg dose infused over 90 min.
Levofloxacin pharmacokinetics are linear and predictable after single and multiple oral or IV dosing regimens. Steady-state conditions are reached within 48 hrs following a 500- or 750-mg once-daily dosage regimen. The mean ±SD peak and trough plasma concentrations attained following multiple once-daily oral dosage regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 μg/mL after the 500-mg doses, and 8.6 ± 1.9 and 1.1 ± 0.4 μg/mL after the 750-mg doses, respectively. The mean ±SD peak and trough plasma concentrations attained following multiple once-daily i.v. regimens were approximately 6.4 ± 0.8 and 0.6 ± 0.2 μg/mL after 500-mg doses, and 12.1 ± 4.1 and 1.3 ± 0.71 μg/mL after 750-mg doses, respectively.
Oral administration with food slightly prolongs the time to peak concentration by approximately 1 hr and slightly decreases the peak concentration by approximately 14%. Therefore, levofloxacin can be administered without regard to food.
The plasma concentration profile of levofloxacin after i.v. administration is similar and comparable in extent of exposure (AUC) to that observed for levofloxacin tablets when equal doses (mg/mg) are administered. Therefore, the oral and i.v. routes of administration can be considered interchangeable.
Distribution: The mean volume of distribution of levofloxacin generally ranges from 74-112 L after single and multiple 500- or 750-mg doses, indicating widespread distribution into body tissues. Levofloxacin reaches its peak levels in skin tissues and in blister fluid of healthy subjects at approximately 3 hrs after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple once-daily oral administration of 750- and 500-mg levofloxacin, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2- to 5-fold higher than plasma concentrations and ranged from approximately 2.4-11.3 μg/g over a 24-hr period after a single 500-mg oral dose.
In vitro, over a clinically relevant range (1-10 μg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24-38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Metabolism: Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered as unchanged drug in urine within 48 hrs, whereas <4% of the dose was recovered in feces in 72 hrs. Less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Excretion: Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6-8 hrs following single or multiple doses of levofloxacin given orally or intravenously. The mean apparent total body clearance and renal clearance range from approximately 144-226 mL/min and 96-142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid results in approximately 24% and 35% reduction in the levofloxacin renal clearance; respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving levofloxacin.
Special Populations: Geriatric: There are no significant differences in levofloxacin pharmacokinetics between young and elderly subjects when the subjects’ differences in creatinine clearance are taken into consideration. Following a 500-mg oral dose of levofloxacin to healthy elderly subjects (66-80 years), the mean terminal plasma elimination half-life of levofloxacin was about 7.6 hrs, as compared to approximately 6 hrs in younger adults. The difference was attributable to the variation in renal function status of the subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by age. Levofloxacin dose adjustment based on age alone is not necessary.
Pediatric: The pharmacokinetics of levofloxacin in pediatric subjects have not been studied.
Gender: There are no significant differences in levofloxacin pharmacokinetics between male and female subjects when subjects’ differences in creatinine clearance are taken into consideration. Following a 500-mg oral dose of levofloxacin to healthy male subjects, the mean terminal plasma elimination half-life of levofloxacin was about 7.5 hrs, as compared to approximately 6.1 hrs in female subjects. This difference was attributable to the variation in renal function status of the male and female subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by the gender of the subjects. Dose adjustments based on gender alone is not necessary.
Race: The effect of race on levofloxacin pharmacokinetics was examined through a covariate analysis performed on data from 72 subjects: 48 white and 24 nonwhite. The apparent total body clearance and apparent volume of distribution were not affected by the race of the subjects.
Renal Insufficiency: Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in patients with impaired renal function (creatinine clearance <50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of levofloxacin are not required following hemodialysls or CAPD. (See Precautions and Dosage & Administration.)
Hepatic Insufficiency: Pharmacokinetic studies in hepatically impaired patients have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment.
Bacterial Infection: The pharmacokinetics of levofloxacin in patients with serious community-acquired bacterial infections are comparable to those observed in healthy subjects.
Drug-Drug Interactions: The potential for pharmacokinetic drug interactions between levofloxacin and theophylline, warfarin, cyclosporine, digoxin, probenecid, cimetidine, sucralfate and antacids has been evaluated. (See Interactions.)
Electrocardiogram: In a study of 48 healthy volunteers receiving single doses of levofloxacin 500, 1000 and 1500 mg and placebo, a dose-related increase from baseline to post-dose of average QTc was observed. These changes were not statistically significant from placebo for the 500-mg dose, variably statistically significant for the 1000-mg dose depending on the correction method used and statistically significant for the 1500-mg dose (see Precautions).
Microbiology: Cravit is a broad-spectrum quinolone antibacterial agent containing levofloxacin, optically active (-)-S-form of racemate ofloxacin synthesized by Daiichi Pharmaceutical Co., Ltd. Cravit shows broad and potent antibacterial activities against gram-positive bacteria, e.g. Staphylococcus sp, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus hemolyticus, Enterococcus sp, and gram-negative bacteria, e.g. E. coli, Klebsiella, Serratia, and Proteus spp, Pseudomonas aeruginosa, Haemophilus influenzae. Moreover, Cravit has antibacterial activities against Peptostreptococcus sp of anaerobic bacteria and Chlamydia trachomatis.
Cravit FC tab 250 mg x 10’s. 500 mg x 10’s. Cravit IV infusion 250 mg/50 mL x 1’s. 500 mg/100 mL x 1’s.
Treatment of adults (more than or equal to 18 years) with mild, moderate and severe infections caused by susceptible strains of the designated microorganisms In the conditions listed as follows. Cravit injection is indicated when i.v. administration offers a route of administration advantageous to the patient (e.g., patient cannot tolerate an oral dosage form). (See Dosage & Administration for specific recommendations.)
Acute maxillary sinusitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis.
Acute bacterial exacerbation of chronic bronchitis due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Community-acquired pneumonia due to Staphylococcus aureus, Streptococcus pneumoniae, Legionella pneumophila or Mycoplasma pneumoniae.
Uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections due to Staphylococcus aureus, or Streptococcus pyogenes.
Complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa.
Acute pyelonephritis (mild to moderate) caused by Escherichia coli.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to levofloxacin. Therapy with levofloxacin may be initiated before results of these tests are known, once results become available, appropriate therapy should be selected.
As with other drugs in the class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with levofloxacin. Culture and susceptibility testing performed periodically during therapy will provide information about the continued susceptibility of the pathogens to the antimicrobial agent and also the possible emergence of bacterial resistance.
Copyright MIMS
Cravit FC Tab: For adults, 100 mg (1 tab) of levofloxacin is usually administered orally 2-3 times a day. The dosage may be adjusted according to the kind of infection and symptoms. In a severe case or insufficient efficacy, 200 mg (2 tab) of levofloxacin is administered 3 times a day.
Usual Dose: 250 or 500 mg administered by slow infusion over 60 min every 24 hrs or 750 mg administered orally or by slow infusion over 90 min every 24 hrs, as indicated by infection and described in the following dosing chart. These recommendations apply to patients with normal renal function (ie, creatinine clearance >80 mL/min). For patients with altered renal function, see Table 2. Oral doses should be administered at least 2 hrs before or 2 hrs after antacids containing magnesium, aluminum, as well as sucralfate, metal cations eg, iron and multivitamin preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution.
Careful administration should be given to patients with severe renal disorders, history of convulsive diseases eg, epilepsy (convulsions may possibly occur), history of hypersensitivity to quinolone antibacterial agents and the elderly.
Cravit IV Infusion: Cravit injection should only be administered by IV infusion. It is not for IM, intrathecal, intraperitoneal or SC administration.
Note: Rapid or bolus IV infusion must be avoided. Levofloxacin injection should be infused IV slowly over a period of not less than 60 or 90 min, depending on the dosage (see Precautions).
Patients with Normal Renal Function: See Table 1.

Patients with Impaired Renal Function: See Table 2.

When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.
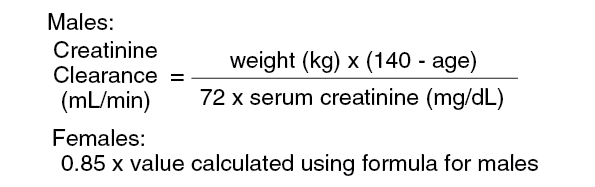
The serum creatinine should represent a steady state of renal function.
Copyright MIMS
Levofloxacin exhibits a low potential for acute toxicity. Mice, rats, dogs and monkeys exhibited the following clinical signs after receiving a single high dose of levofloxacin: Ataxia, ptosis, decreased locomotor activity, dyspnea, prostration, tremors and convulsions. Doses in excess of 1500 mg/kg orally and 250 mg/kg i.v. produced significant mortality in rodents. In the event of an acute overdosage, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Levofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Copyright MIMS
Persons with a history of hypersensitivity to levofloxacin, quinolone antimicrobial agents, or any other components of Cravit.
Copyright MIMS
Fluoroquinolones, including Levofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
Patients experiencing pain, swelling, inflammation of a tendon or tendon rupture should be advised to stop taking Levofloxacin and to contact their health care professional promptly about changing their antimicrobial therapy. Patients should also avoid exercise and using the affected area at the first sign of tendon pain, swelling, or inflammation. Tendon rupture can occur during or after completion of therapy; cases occuring up to several months after completion of therapy have been reported.
Exacerbation or myasthenia gravis: Fluoroquinolones, including levofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in person with myasthenia gravis.
Post marketing serious adverse events, including deaths and requirement for ventilator support have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid levofloxacin in patients with known history of myasthenia gravis.
The safety and efficacy of levofloxacin in children, adolescents (<18 years), pregnant and nursing women have not been established (see Use in pregnancy, Use in lactation under Use in Pregnancy & Lactation and Use in children under Precautions).
In immature rats and dogs, the oral and i.v. administration of levofloxacin increased the incidence and severity of osteochondrosis. Other fluoroquinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species (see Pharmacology under Actions).
Convulsions and toxic psychoses have been reported in patients receiving quinolones, including levofloxacin. Quinolones may also cause increased intracranial pressure and central nervous system stimulation which may lead to tremors, restlessness, anxiety, lightheadedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia and rarely, suicidal thoughts or acts. These reactions may occur following the 1st dose. If these reactions occur in patients receiving levofloxacin, the drug should be discontinued and appropriate measures instituted. As with other quinolones, levofloxacin should be used with caution in patients with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction) (see Information for Patients under Precautions, Interactions and Adverse Reactions).
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones including levofloxacin. These reactions often occur following the 1st dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/ swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching and other serious skin reactions. Levofloxacin should be discontinued immediately at the first appearance of a skin rash or any other signs of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, i.v. fluids, antihistamines, corticosteroids, pressor amines and airway management, as clinically indicated (see Precautions and Adverse Reactions).
Serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones including levofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following: fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome); vasculitis; arthralgia; myalgia; serum sickness; allergic pneumonitis; interstitial nephritis; acute renal insufficiency or failure; hepatitis; jaundice; acute hepatic necrosis or failure; anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities. Cravit should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity and supportive measures instituted (see Information for Patients under Precautions and Adverse Reactions).
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including levofloxacin, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug clinically effective against C. difficile colitis (see Adverse Reactions).
Copyright MIMS
Use in pregnancy: There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies potential risk to the fetus (see Warnings).
Use in lactation: Levofloxacin has not been measured in human milk. Based upon data from ofloxacin, it can be presumed that levofloxacin will be excreted in human milk. Because of the potential for serious adverse reactions from levofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
Copyright MIMS
Cravit FC Tab: For adults, 100 mg (1 tab) of levofloxacin is usually administered orally 2-3 times a day. The dosage may be adjusted according to the kind of infection and symptoms. In a severe case or insufficient efficacy, 200 mg (2 tab) of levofloxacin is administered 3 times a day.
Usual Dose: 250 or 500 mg administered by slow infusion over 60 min every 24 hrs or 750 mg administered orally or by slow infusion over 90 min every 24 hrs, as indicated by infection and described in the following dosing chart. These recommendations apply to patients with normal renal function (ie, creatinine clearance >80 mL/min). For patients with altered renal function, see Table 2. Oral doses should be administered at least 2 hrs before or 2 hrs after antacids containing magnesium, aluminum, as well as sucralfate, metal cations eg, iron and multivitamin preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution.
Careful administration should be given to patients with severe renal disorders, history of convulsive diseases eg, epilepsy (convulsions may possibly occur), history of hypersensitivity to quinolone antibacterial agents and the elderly.
Cravit IV Infusion: Cravit injection should only be administered by IV infusion. It is not for IM, intrathecal, intraperitoneal or SC administration.
Note: Rapid or bolus IV infusion must be avoided. Levofloxacin injection should be infused IV slowly over a period of not less than 60 or 90 min, depending on the dosage (see Precautions).
Patients with Normal Renal Function: See Table 1.

Patients with Impaired Renal Function: See Table 2.

When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance: See equation.
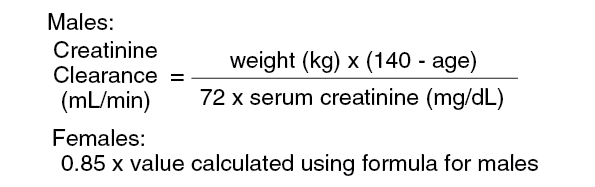
The serum creatinine should represent a steady state of renal function.
Copyright MIMS
General: Because a rapid or bolus i.v. injection may result in hypotension, levofloxacin injection should only be administered by slow i.v, infusion over a period of 60 or 90 min depending on the dosage (see Dosage & Administration).
Although levofloxacin is more soluble than other quinolones, adequate hydration of patients receiving levofloxacin should be maintained to prevent the formation of a highly concentrated urine.
Administer levofloxacin with caution in the presence of renal insufficiency. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced. In patients with impaired renal function (creatinine clearance <50 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance. (See Pharmacology: Pharmacokinetics under Actions and Dosage & Administration.)
Moderate to severe phototoxicity reactions have been observed in patients exposed to direct sunlight while receiving drugs in this class. Excessive exposure to sunlight should be avoided. However, in clinical trials with levofloxacin, phototoxicity has been observed in <0.1% of patients. Therapy should be discontinued if phototoxicity (e.g., a skin eruption) occurs.
As with other quinolones, levofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction). (See Warnings and Interactions.)
As with other quinolones, disturbances of blood glucose, including symptomatic hyper- and hypoglycemia, have been reported, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide/glibenclamide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. If a hypoglycemic reaction occurs in a patient being treated with levofloxacin, levofloxacin should be discontinued immediately and appropriate therapy should be initiated immediately. (See Interactions and Adverse Reactions.)
Some quinolones, including levofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram (see Pharmacology: Electrocardiogram under Actions) and infrequent cases of arrhythmia. During post-marketing surveillance, very rare cases of torsades de pointes have been reported in patients taking levofloxacin. These reports generally involved patients with concurrent medical conditions or concomitant medications that may have been contributory. The risk of arrhythmias may be reduced by avoiding concurrent use with other drugs that prolong the QT interval including class Ia or class III antiarrhythmic agents; In addition, use of levofloxacin in the presence of risk factors for torsades de pointes such as hypokalemia, significant bradycardia, and cardiomyopathy should be avoided.
As with any potent antimicrobial drug, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during therapy. (See Warnings and Adverse Reactions.)
Information for Patients: Patients should be advised: to drink fluids liberally; that antacids containing magnesium or aluminum, as well as sucralfate, metal cations, e.g. iron, and multivitamin preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution should be taken at least 2 hrs before or 2 hrs after oral levofloxacin administration (see Interactions); that oral levofloxacin can be taken without regard to meals; to discontinue treatment and inform the physician if the patient experiences pain, inflammation or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded; that levofloxacin may be associated with hypersensitivity reactions, even following the 1st dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (e.g., swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction (see Warnings and Adverse Reactions); to avoid excessive sunlight or artificial ultraviolet light while receiving levofloxacin and to discontinue therapy if phototoxicity (i.e., skin eruption) occurs; that if diabetic and being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, levofloxacin should be discontinued and consult a physician (see Interactions); that concurrent administration of warfarin and levofloxacin has been associated with increases of the International Normalized Ratio (INR) or prothrombin time and clinical episodes of bleeding. Patients should notify their physician if they are taking warfarin; that convulsions have been reported in patients taking quinolones, including levofloxacin and to notify their physician before taking Cravit if there is a history of this condition.
Effects on the Ability to Drive or Operate Machinery: Patients should be advised that levofloxacin may cause neurologic adverse effects (e.g., dizziness, lightheadedness) and they should know how to react to levofloxacin before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination (see Warnings and Adverse Reactions).
Carcinogenicity, Mutagenicity & Impairment of Fertility: In a lifetime bioassay in rats, levofloxacin exhibited no carcinogenic potential following daily dietary administration for 2 years; the highest dose (100 mg/kg/day) was 1.4 times the highest recommended human dose (750 mg) based upon relative body surface area.
Levofloxacin was not mutagenic in the following assays: Ames bacterial mutation assay (S. typhimurium and E. coli), CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and sister chromatid exchange (CHL/IU cell line) assays.
Levofloxacin caused no impairment of fertility or reproductive performance in rats at oral doses as high as 360 mg/kg/day, corresponding to 4.2 times the highest recommended human dose based upon relative body surface area and i.v. doses as high as 100 mg/kg/day, corresponding to 1.2 times the highest recommended human dose based upon relative body surface area.
Teratogenicity: Pregnancy Category C: Levofloxacin was not teratogenic in rats at oral doses as high as 810 mg/kg/day, which corresponds to 9.4 times the highest recommended human dose based upon relative body surface area or at i.v. doses as high as 160 mg/kg/day, corresponding to 1.9 times the highest recommended human dose based upon relative body surface area. The oral dose of 810 mg/kg/day to rats caused decreased fetal body weight and increased fetal mortality. No teratogenicity was observed when rabbits were dosed orally as high as 50 mg/kg/day, which corresponds to 1.1 times the highest recommended human dose based upon relative body surface area or when dosed intravenously as high as 25 mg/kg/day, corresponding to 0.5 times the highest recommended human dose based upon relative body surface area.
Use in pregnancy: There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies potential risk to the fetus (see Warnings).
Use in lactation: Levofloxacin has not been measured in human milk. Based upon data from ofloxacin, it can be presumed that levofloxacin will be excreted in human milk. Because of the potential for serious adverse reactions from levofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
Use in children: Safety and effectiveness in pediatric patients and adolescents <18 years have not been established. Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species (see Warnings.)
Use in the elderly: In phase 3 clinical trials, 1190 levofloxacin-treated patients (25%) were more than or equal to 65 years. Of these, 675 patients (14%) were between 65 and 74 years and 515 patients (11%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However, since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to levofloxacin may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
Copyright MIMS
The incidence of drug-related adverse reactions in patients during Phase 3 clinical trials conducted in North America was 6.3%. Among patients receiving levofloxacin therapy, 3.9% discontinued levofloxacin therapy due to adverse experiences. The overall incidence, type and distribution of adverse events were similar in patients receiving levofloxacin doses of 750 mg once daily compared to patients receiving doses from 250 mg once daily to 500 mg twice daily.
In clinical trials, the following events were considered likely to be drug-related in patients receiving levofloxacin: nausea 1.3%, diarrhea 1%, vaginitis 0.7%, insomnia 0.5%, abdominal pain 0.4%, flatulence 0.4%, pruritus 0.4%, dizziness 0.3%, dyspepsia 0.3%, rash 0.3%, genital moniliasis 0.2%, taste perversion 0.2%, vomiting 0.2%, constipation 0.1%, fungal infection 0.1%, genital pruritis 0.1%, headache 0.1%, moniliasis 0.1%, nervousness 0.1%, erythematous rash 0.1%, urticaria 0.1%.
In clinical trials, the following events occurred in >3%, regardless of drug relationship: Nausea 7.2%, headache 6.4%, diarrhea 5.6%, insomnia 4.6%, injection site reaction 3.5% and constipation 3.2%.
In clinical trials, the following events occurred in 1-3% of patients, regardless of drug relationship: Dizziness 2.7%, abdominal pain 2.5%, dyspepsia 2.4%, vomiting 2.3%, vaginitis 1.8%, injection site pain 1.7%, flatulence 1.5%, pain 1.4%, pruritus 1.3%, sinusitis 1.3%, chest pain 1.2%, fatigue 1.2%, rash 1.2%, back pain 1.1%, injection site inflammation 1.1%, rhinitis 1% and taste perversion 1%.
In clinical trials, the following events, of potential medical importance, occurred at a rate of <1%, regardless of drug relationship: Autonomic Nervous System Disorders: Postural hypotension.
Body as a Whole-General Disorders: Asthenia, edema, fever, malaise, rigors, substernal chest pain and syncope.
General Cardiovascular Disorders: Cardiac failure, circulatory failure, hypertension and hypotension.
Central and Peripheral Nervous System Disorders: Abnormal coordination, coma, convulsions (seizures), hyperkinesia, hypertonia, hypoesthesia, involuntary muscle contractions, paresthesia, paralysis, speech disorder, stupor, tremor and vertigo.
Gastrointestinal System Disorders: Dry mouth, dysphagia, gastroenteritis, G.I. hemorrhage, pancreatitis, pseudomembranous colitis and tongue edema.
Hearing and Vestibular Disorders: Ear disorder (not otherwise specified) and tinnitus.
Heart Rate and Rhythm Disorders: Arrhythmia, atrial fibrillation, bradycardia, cardiac arrest, heart block, palpitation, supraventricular tachycardia, tachycardia and ventricular fibrillation.
Liver and Biliary System Disorders: Abnormal hepatic function, cholelithiasis, hepatic coma and jaundice.
Metabolic and Nutritional Disorders: Aggravated diabetes mellitus, dehydration, hyperglycemia, hyperkalemia, hypoglycemia, hypokalemia, increased LDH and decreased weight.
Musculoskeletal System Disorders: Arthralgia, arthritis, arthrosis, muscle weakness, myalgia, osteomyelitis, rhabdomyolysis, synovitis and tendinitis.
Myo-, Endo-, Pericardial and Valve Disorders: Angina pectoris, coronary thrombosis and myocardial infarction.
Neoplasms: Carcinoma.
Other Special Senses Disorders: Parosmia.
Platelet, Bleeding and Clotting Disorders: Abnormal platelets, embolism (blood clot), epistaxis, purpura and thrombocytopenia.
Psychiatric Disorders: Abnormal dreaming, aggressive reaction, agitation, anorexia, anxiety, confusion, delirium, depression, emotional lability, hallucination, impaired concentration, impotence, manic reaction, mental deficiency, nervousness, paranoia, sleep disorder, somnolence and withdrawal syndrome.
Red Blood Cell Disorders: Anemia.
Reproductive Disorders: Ejaculation failure.
Resistance Mechanism Disorders: Fungal infection and genital moniliasis.
Respiratory System Disorders: ARDS, asthma, coughing, dyspnea, haemoptysis, hypoxia, pleural effusion and respiratory insufficiency.
Skin and Appendages Disorders: Erythema nodosum, genital pruritus, increased sweating, skin disorder, skin exfoliation, skin ulceration and urticaria.
Urinary System Disorders: Abnormal renal function, acute renal failure, face edema and haematuria.
Vascular (Extracardiac) Disorders: Cerebrovascular disorder and phlebitis.
Vision Disorders: Abnormal vision, conjunctivitis and diplopia.
White Cell and RES Disorders: Granulocytopenia, leukocytosis, leukopenia, lymphadenopathy, abnormal WBC (not otherwise specified).
In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with other quinolones. The relationship of the drugs to these events is not presently established.
Crystalluria and cylindruria have been reported with other quinolones.
The following laboratory abnormalities appeared in 2.2% of patients receiving levofloxacin. It is not known whether these abnormalities were caused by the drug or the underlying condition being treated.
Blood Chemistry: Decreased glucose.
Hematology: Decreased lymphocytes.
Post-Marketing Adverse Reactions: Additional adverse events reported from worldwide post-marketing experience with levofloxacin include: Allergic pneumonitis, anaphylactic shock, anaphylactoid reaction, dysphonia, abnormal EEG, encephalopathy, eosinophilia, erythema multiforme, hemolytic anemia, multisystem organ failure, increased International Normalized Ratio (INR)/prothrombin time, Stevens-Johnson syndrome, tendon rupture, torsade de pointes and vasodilation, Exacerbation of myasthenia gravis.
Copyright MIMS
Antacids, Sucralfate, Metal Cations, Multivitamins: Tablet: While the chelation by divalent cations is less marked than with other quinolones, concurrent administration of Cravit tablets with antacids containing magnesium or aluminum, as well as sucralfate, metal cations, such as iron, and multivitamin preparations with zinc, may interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. Tablets with antacids containing magnesium, aluminum, as well as sucralfate, metal cations, such as iron and multivitamins preparations with zinc or didanosine (Videx) chewable/buffered tablets or the pediatric powder for oral solution may substantially interfere with the gastrointestinal absorption of levofloxacin, resulting in systemic levels considerably lower than desired. These agents should be taken at least 2 hrs before or 2 hrs after levofloxacin administration.
Injection: There are no data concerning an interaction of i.v. quinolones with oral antacids, sucralfate, multivitamins, didanosine (Videx), or metal cations. However, no quinolone should be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same i.v. line. (See Dosage & Administration.)
Theophylline: No significant effect of levofloxacin on the plasma concentrations, AUC and other disposition parameters for theophylline was detected in a clinical study involving 14 healthy volunteers. Similarly, no apparent effect of theophylline on levofloxacin absorption and disposition was observed. However, concomitant administration of other quinolones with theophylline has resulted in prolonged elimination half-life, elevated serum theophylline levels, and a subsequent increase in the risk of theophylline-related adverse reactions in the patient population. Therefore, theophylline levels should be closely monitored and appropriate dosage adjustments made when levofloxacin is co-administered. Adverse reactions, including seizures, may occur with or without an elevation in serum theophylline levels. (See Warnings and General under Precautions.)
Warfarin: No significant effect of levofloxacin on the peak plasma concentrations, AUC, and other disposition parameters for R- and S-warfarin was detected in a clinical study involving healthy volunteers. Similarly, no apparent effect of warfarin on levofloxacin absorption and disposition was observed. There have been reports during the post-marketing experience in patients that levofloxacin enhances the effects of warfarin. Elevations of the prothrombin time in the setting of concurrent warfarin and levofloxacin use have been associated with episodes of bleeding. Prothrombin time, International Normalized Ratio (INR) or other suitable anticoagulation tests should be closely monitored if levofloxacin is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding.
Cyclosporine: No significant effect of levofloxacin on the peak plasma concentrations, AUC and other disposition parameters for cyclosporine was detected in a clinical study involving healthy volunteers. However, elevated serum levels of cyclosporine have been reported in the patient population when co-administered with some other quinolones. Levofloxacin Cmax and Ke were slightly lower while Tmax and t½ were slightly longer in the presence of cyclosporine than those observed in other studies without concomitant medication. The differences, however, are not considered to be clinically significant. Therefore, no dosage adjustment is required for levofloxacin or cyclosporine when administered concomitantly.
Digoxin: No significant effect of levofloxacin on the peak plasma concentrations, AUC and other disposition parameters for digoxin was detected in a clinical study involving healthy volunteers. Levofloxacin absorption and disposition kinetics were similar in the presence or absence of digoxin. Therefore, no dosage adjustment for levofloxacin or digoxin is required when administered concomitantly.
Probenecid and Cimetidine: No significant effect of probenecid or cimetidine on the rate and extent of levofloxacin absorption was observed in a clinical study involving healthy volunteers. The AUC and t½ of levofloxacin were 27-38% and 30% higher, respectively, while CL/F and CLR were 21-35% lower during concomitant treatment with probenecid or cimetidine compared to levofloxacin alone. Although these differences were statistically significant, the changes were not high enough to warrant dosage adjustment for levofloxacin when probenecid or cimetidine is co-administered.
Non-steroidal anti-inflammatory drugs: The concomitant administration of a nonsteroidal anti-inflammatory drug with a quinolone, including levofloxacin, may increase the risk of CNS stimulation and convulsive seizures. (See Warnings and General under Precautions.)
Antidiabetic Agents: Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with quinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered.
Copyright MIMS
Inspect visually for particulate matter prior to administration. Samples containing visible particles should be discarded. Once the infusion bottle has been opened (rubber stopper perforated) the solution should be used immediately (within 3 hours) in order to prevent any bacterial contamination.
Compatibilities/Incompatibilities: Since Cravit i.v. solution is ready for use, it may be given alone or with one of the following solutions: 0.9% Sodium chloride solution, 5% dextrose injection, 2.5% dextrose in Ringer solution and combination solutions for parenteral nutrition (amino acids, carbohydrates, electrolytes).
Cravit i.v. solution for infusion should not be mixed with certain other solutions (e.g., sodium hydrogen carbonate) or with heparin.
Copyright MIMS
Store below 30°C in well-closed containers. Protect from light.
Use within 3 days after removal from the carton.
Copyright MIMS
Each film-coated tablet of CRAVIT 250mg contains 250mg of levofloxacin as active ingredient corresponding to 256.23mg of levofloxacin hemihydrate and each film-coated tablet of CRAVIT 500mg contains 500mg of levofloxacin as active ingredient corresponding to 512.46mg of levofloxacin hemihydrate.
Cravit i.v. 5mg/ml is a sterile clear greenish-yellow solution for intravenous administration. Each 50ml bottle contains 250mg of levofloxacin as active substance. Each 100ml bottle contains 500mg of levofloxacin as active substance.
The solution also contains the following ingredients: sodium chloride; sodium hydroxide; hydrochloric acid (qs: pH 4.8) and water for injection (Na+ concentration: 154mmol/L).
CRAVIT (levofloxacin) Tablets/Injection are synthetic broad spectrum antibacterial agents for oral and intravenous administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The chemical name is (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-5-carboxylic acid hemihydrate.
Its empirical formula of C18H20FN3O4·½H2O and its molecular weight is 370.38. Levofloxacin is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine. The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin is essentially constant (approximately 100 mg/mL). Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9. Levofloxacin has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Al+3>Cu+2>Zn+2>Mg+2>Ca+2.
Copyright MIMS
Synthetic broad-spectrum antibacterial agent for oral and i.v. administration.
Pharmacology: The main mechanism of action of levofloxacin is inhibition of DNA gyrase. It is 2-fold stronger than that of ofloxacin. There is not much difference between MIC and MBC. The activity of levofloxacin is bactericidal. In the observation of bacterial morphology, bacteriolysis can be seen in the concentration around MIC.
Cravit shows clinical efficacy on respiratory tract infections, genitourinary tract infections, biliary tract infections, intestinal tract infections and other various infections in the surgical, gynecological, dermatological, otorhinolaryngological, ophthalmological, and dental and oral surgery fields.
Pharmacokinetics: Absorption: Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained 1-2 hrs after oral dosing. The absolute bioavailability of a 500- and 750-mg tablet of levofloxacin are both approximately 99%, demonstrating complete oral absorption of levofloxacin. Following a single i.v. dose of levofloxacin to healthy volunteers, the mean ±SD peak plasma concentration attained was 6.2 ± 1.0 μg/mL after a 500-mg dose infused over 60 min and 11.5 ± 4.0 μg/mL after a 750-mg dose infused over 90 min.
Levofloxacin pharmacokinetics are linear and predictable after single and multiple oral or IV dosing regimens. Steady-state conditions are reached within 48 hrs following a 500- or 750-mg once-daily dosage regimen. The mean ±SD peak and trough plasma concentrations attained following multiple once-daily oral dosage regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 μg/mL after the 500-mg doses, and 8.6 ± 1.9 and 1.1 ± 0.4 μg/mL after the 750-mg doses, respectively. The mean ±SD peak and trough plasma concentrations attained following multiple once-daily i.v. regimens were approximately 6.4 ± 0.8 and 0.6 ± 0.2 μg/mL after 500-mg doses, and 12.1 ± 4.1 and 1.3 ± 0.71 μg/mL after 750-mg doses, respectively.
Oral administration with food slightly prolongs the time to peak concentration by approximately 1 hr and slightly decreases the peak concentration by approximately 14%. Therefore, levofloxacin can be administered without regard to food.
The plasma concentration profile of levofloxacin after i.v. administration is similar and comparable in extent of exposure (AUC) to that observed for levofloxacin tablets when equal doses (mg/mg) are administered. Therefore, the oral and i.v. routes of administration can be considered interchangeable.
Distribution: The mean volume of distribution of levofloxacin generally ranges from 74-112 L after single and multiple 500- or 750-mg doses, indicating widespread distribution into body tissues. Levofloxacin reaches its peak levels in skin tissues and in blister fluid of healthy subjects at approximately 3 hrs after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple once-daily oral administration of 750- and 500-mg levofloxacin, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2- to 5-fold higher than plasma concentrations and ranged from approximately 2.4-11.3 μg/g over a 24-hr period after a single 500-mg oral dose.
In vitro, over a clinically relevant range (1-10 μg/mL) of serum/plasma levofloxacin concentrations, levofloxacin is approximately 24-38% bound to serum proteins across all species studied, as determined by the equilibrium dialysis method. Levofloxacin is mainly bound to serum albumin in humans. Levofloxacin binding to serum proteins is independent of the drug concentration.
Metabolism: Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered as unchanged drug in urine within 48 hrs, whereas <4% of the dose was recovered in feces in 72 hrs. Less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Excretion: Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6-8 hrs following single or multiple doses of levofloxacin given orally or intravenously. The mean apparent total body clearance and renal clearance range from approximately 144-226 mL/min and 96-142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid results in approximately 24% and 35% reduction in the levofloxacin renal clearance; respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving levofloxacin.
Special Populations: Geriatric: There are no significant differences in levofloxacin pharmacokinetics between young and elderly subjects when the subjects’ differences in creatinine clearance are taken into consideration. Following a 500-mg oral dose of levofloxacin to healthy elderly subjects (66-80 years), the mean terminal plasma elimination half-life of levofloxacin was about 7.6 hrs, as compared to approximately 6 hrs in younger adults. The difference was attributable to the variation in renal function status of the subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by age. Levofloxacin dose adjustment based on age alone is not necessary.
Pediatric: The pharmacokinetics of levofloxacin in pediatric subjects have not been studied.
Gender: There are no significant differences in levofloxacin pharmacokinetics between male and female subjects when subjects’ differences in creatinine clearance are taken into consideration. Following a 500-mg oral dose of levofloxacin to healthy male subjects, the mean terminal plasma elimination half-life of levofloxacin was about 7.5 hrs, as compared to approximately 6.1 hrs in female subjects. This difference was attributable to the variation in renal function status of the male and female subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by the gender of the subjects. Dose adjustments based on gender alone is not necessary.
Race: The effect of race on levofloxacin pharmacokinetics was examined through a covariate analysis performed on data from 72 subjects: 48 white and 24 nonwhite. The apparent total body clearance and apparent volume of distribution were not affected by the race of the subjects.
Renal Insufficiency: Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in patients with impaired renal function (creatinine clearance <50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of levofloxacin are not required following hemodialysls or CAPD. (See Precautions and Dosage & Administration.)
Hepatic Insufficiency: Pharmacokinetic studies in hepatically impaired patients have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment.
Bacterial Infection: The pharmacokinetics of levofloxacin in patients with serious community-acquired bacterial infections are comparable to those observed in healthy subjects.
Drug-Drug Interactions: The potential for pharmacokinetic drug interactions between levofloxacin and theophylline, warfarin, cyclosporine, digoxin, probenecid, cimetidine, sucralfate and antacids has been evaluated. (See Interactions.)
Electrocardiogram: In a study of 48 healthy volunteers receiving single doses of levofloxacin 500, 1000 and 1500 mg and placebo, a dose-related increase from baseline to post-dose of average QTc was observed. These changes were not statistically significant from placebo for the 500-mg dose, variably statistically significant for the 1000-mg dose depending on the correction method used and statistically significant for the 1500-mg dose (see Precautions).
Microbiology: Cravit is a broad-spectrum quinolone antibacterial agent containing levofloxacin, optically active (-)-S-form of racemate ofloxacin synthesized by Daiichi Pharmaceutical Co., Ltd. Cravit shows broad and potent antibacterial activities against gram-positive bacteria, e.g. Staphylococcus sp, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus hemolyticus, Enterococcus sp, and gram-negative bacteria, e.g. E. coli, Klebsiella, Serratia, and Proteus spp, Pseudomonas aeruginosa, Haemophilus influenzae. Moreover, Cravit has antibacterial activities against Peptostreptococcus sp of anaerobic bacteria and Chlamydia trachomatis.
Copyright MIMS
Copyright MIMS
Cravit FC tab 250 mg x 10’s. 500 mg x 10’s. Cravit IV infusion 250 mg/50 mL x 1’s. 500 mg/100 mL x 1’s.
Copyright MIMS






