The Role of Levofloxacin in Treating Urinary Tract Infections
Professor George A. Richard, MD,
Department of Pediatrics,
Nephrology Division,
University of Florida,
Gainesville, FL, USA

Urinary tract infections (UTIs) are so prevalent that at some point in their lives, virtually all women will experience this problem. In fact, they have been reported to add $1 billion to the costs of community care in the USA alone (1). These infections are associated with significant morbidity, resulting in tremendous costs to the patient and society, and therefore, any antimicrobial agent which can help reduce these costs will provide the physician with significant advantages. Agents which possess wide antibacterial activity, the option of using once-daily dosing schedules and are well tolerated would be extremely useful in managing these infections. In addition, if they also maintain activity against the ever-increasing range of resistant pathogens, they would be even more useful. It is fair to say that fluoroquinolones are such agents. They are effective against all UTIs, running the gamut from acute uncomplicated cystitis, recurrent disease, acute uncomplicated pyelonephritis, through to complicated UTI and asymptomatic bacteriuria.
Levofloxacin, one of the new fluoroquinolones, stands out as one of the most effective fluoroquinolones, with excellent pharmacological attributes, clinical efficacy and an unbeatable track record for safety and tolerability. To discuss the role of levofloxacin in managing UTIs, Penetration interviewed Professor George A. Richard, Department of Pediatrics, Nephrology Division, University of Florida, Gainesville, FL, USA, who used his wide clinical and research experience to provide a clear summary of the role of levofloxacin in managing these infections, differences among the individual agents in the class of fluoroquinolones, and the changing face of UTIs today.
![]()
Questions
Q3. How does the activity profile of levofloxacin relate to these pathogens?
Q6. What investigations should be initiated when the patient with UTI is first seen?
Q9. Could you summarize any randomized, comparative trials evaluating fluoroquinolones in UTIs.
Q11. How does levofloxacin compare to comparator agents in terms of tolerability?
Q12. Do you use levofloxacin in preference to ciprofloxacin?
Q13. What is your opinion or impression on differences between levofloxacin and gatifloxacin?
Q15. Do you have any experience with using a higher dose of levofloxacin?
Q19. What differences are there in terms of gender in regard to the incidence of different forms of UTI?
Q20. Do you see a role for fluoroquinolones in prophylaxis to avoid recurrent UTI?
![]()
Answers
Q1. Could you describe the pharmacokinetic and pharmacodynamic features of levofloxacin which make it a useful agent for treating urinary tract infections (UTIs)?
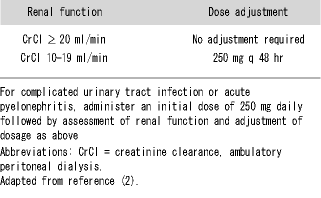
A1. Levofloxacin, the l-isomer of ofloxacin, is 128 times more potent than the d-isomer and possesses a number of excellent pharmacokinetic characteristics. It has extremely high bioavailability, as illustrated by the fact that a 500 mg levofloxacin tablet achieves 100% absorption resulting in a very high Cmax (Table 1) (2). It has extensive tissue distribution and achieves a high concentration in the urine and kidney. For example, after a single 500 mg dose, the concentration in the urine reaches 108 (μg/ml, higher than most comparators, and far exceeding the MIC of all of the most important uropathogens. Another advantageous pharmacokinetic feature is that these parameters do not vary between males and females, nor do they vary with age. However, since levofloxacin is handled by the kidney, with over 80% urinary excretion, dosage must be modified in those with decreased renal function (Table 2) (2).
Pharmacodynamic features are also very good, with levofloxacin possessing concentration-dependent killing, as do all the fluoroquinolones. Thus, the Cmax to MIC (peak/MIC) ratio is one of the most important pharmacodynamic factors, which can be used to predict clinical and microbiological outcome. Research into this has confirmed that a ratio of greater than 12.2 is ideal in UTIs(3), while levofloxacin dosage results in a concentration many times greater than that. Another useful feature possessed by levofloxacin is that it demonstrates a significant postantibiotic effect, with suppression of the organisms maintained between doses (4, 5). Thus, I believe it is fair to say that levofloxacin has a very favorable pharmacokinetic and pharmacodynamic profile, achieving very high kidney urine concentrations for eradication of most of the organisms that cause UTIs.
Table 1. Pharmacokinetic variables associated with levofloxacin therapya
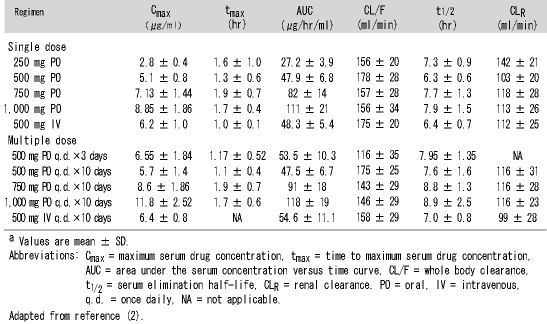
Table 2. Dosage recommendations of levofloxacin for patients with renal dysfunction
Q2. What are the major pathogens involved in the different forms of UTI – acute uncomplicated through to chronic complicated forms of disease?
A2. The pathogens responsible for UTIs differ according to the type of infections and also between different types of patients (6). In acute uncomplicated cystitis, most studies have demonstrated the overwhelming pathogenicity of Escherichia coli, which accounts for 70-95% of infection. The remaining 5-20% in the 18-25-year old age group are caused by Staphylococcus saprophyticus (coagulase-negative staphylococci), with all other pathogens reported to be associated with less than 1% of infections.
In acute uncomplicated pyelonephritis, E. coli remains predominant, responsible for approximately 85% of infections, with another 4% due to Proteus mirabilis and Klebsiellaspp., respectively. Aerococcus spp. and Enterobacter spp. cause less than 1% each, and there is a mixed group of around 5%. In acute uncomplicated pyelonephritis, it is rare to identify S. saprophyticus as the causative pathogen. Acute pyelonephritis usually occurs in young women, but there is another group that develop this problem, namely the elderly. They are slightly less likely to have an E. coli infection (60%), and they are more likely to have infections caused by resistant isolates. Proteus spp., Pseudomonas spp., Klebsiellaspp., and Serratia spp. are also reported in these older patients with acute pyelonephritis.
Complicated UTIs, as expected, are caused by a wider range of pathogens, often related to the underlying problem the patient may have. In these patients, E. coli accounts for 30% of infections, Enterococcus faecalis 22%, Pseudomonas aeruginosa 20%, Klebsiella spp. 5%, P. mirabilis, 4%, and S. saprophyticus 1%. A broader spectrum of antimicrobials are therefore required in complicated UTIs to cover this increased range of pathogens, many of which are more likely to be resistant.
There remain a number of important patient subgroups, depending on other associated factors. One such group is that of asymptomatic bacteriuria, which occurs in 5% of pregnant women. If such women remain untreated, 40% are likely to develop pyuria. A separate risk group are the elderly, who are more likely to develop UTIs, particularly resistant E. coli forms. Catheterized patients and those with renal calculi have a higher incidence of infection caused by P. mirabilis, and patients with abscesses are likely to have E. coli, staphylococcal or Candida infections.
Q3. How does the activity profile of levofloxacin relate to these pathogens?
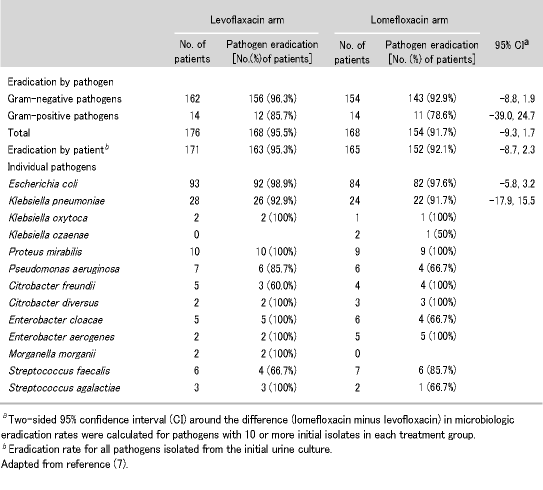
A3. The activity profile of levofloxacin is broad spectrum with excellent in vitro activity against most aerobic Gram-positive and Gram-negative pathogens, including organisms responsible for UTIs such as E. coli, Klebsiella spp., Proteus spp., and other Enterobacteriaceae as well as being effective against S. saprophyticus. This makes it an excellent choice for treating UTIs, especially now with the rising incidence of resistance to other agents. Levofloxacin achieves very high eradication rates for all major uropathogens. In fact, a study performed by Klimberg et al. evaluated the bacterial eradication of levofloxacin in 171 patients compared to 165 treated with lomefloxacin (7). The overall microbiological eradication rate of pathogens for levofloxacin was 95.5% compared with 91.7% for lomefloxacin (Table 3) (7).
Table 3. Rates of eradication of uropathogens in patients with complicated urinary tract infections treated with levofloxacin or lomefloxacin
Q4. You have mentioned increased resistance. Could you comment on the major changes in susceptibility patterns of these urinary pathogens over the last few years? How has this altered the treatment options offered?
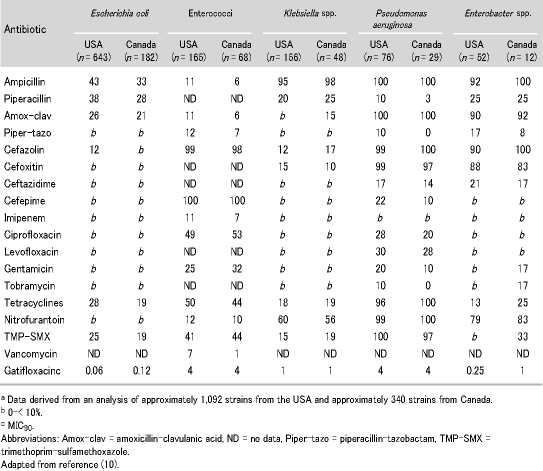
A4. There have definitely been important changes in the sensitivity of uropathogens over the last 15 years which have resulted in a move away from more traditional agents to the new fluoroquinolones. These changes differ among the different forms of UTI, so I will summarize what has happened in each subgroup of infection. First, looking at uncomplicated infections, a recent paper by Gupta et al. has reported that antimicrobial resistance among uropathogens causing community UTIs is increasing (8). The most important factor has been the increase in resistance to trimethoprim-sulfamethoxazole (TMP-SMX). These changes have been apparent for a long time with a 1993 study reported by Stamm et al. showing that a third of causative organisms were resistant to ampicillin, 5-15% were resistant to TMP-SMX, and less than 5% were resistant to fluoroquinolones (9). Results from a 1997 study in the USA and Canada showed that resistance to levofloxacin for all the uropathogens, apart from Pseudomonas spp., was not detectable (Table 4) (10). In 1999, Gupta et al. found that in the period from 1992-1995, 90% of UTIs were caused by either E. coli or S. saprophyticus, with 20% of these resistant to ampicillin, cephalosporin and TMP-SMX (11). The resistance to TMP-SMX has generally been shown to increase from 8-16% over the five-year period. This has led to the increased use of the more efficacious fluoroquinolones rather than ampicillin, amoxicillin, and TMP-SMX, particularly in certain geographic areas where resistance is more predominant.
Changes in the organisms causing complicated UTIs have also been reported, with a 1992 paper by Stamm et al. demonstrating a 70% resistance rate to ampicillin, 37% resistance to TMP-SMX, and 49% to cephalosporins (12). In sharp contrast to this, the resistance rate to fluoroquinolones was less than 5%. In fact, this problem with ampicillin has been well recognized for the past 15 years, with an earlier 1987 study by Stamm et al. reporting reduced efficacy of ampicillin in acute pyelonephritis (13). In this study, ampicillin was compared to TMP-SMX. Results revealed that after two weeks of treatment, 65% of those treated with ampicillin were cured, while TMP-SMX was associated with a 90% cure rate. When six weeks of therapy were given, the figures worsened with a 40% cure rate with ampicillin, and 83% with TMP-SMX. Thus, even in 1987, ampicillin was not an effective agent in UTIs, due to the problem of significant resistance among E. coli. It has become increasingly obvious to clinicians that, when treating UTIs, the possibility of the infection being caused by a resistant pathogen must be considered. Therefore, the increased resistance to ampicillin, TMP-SMX, and cephalosporins in parts of the country has made fluoroquinolones a major player in treating UTIs, especially in the treatment of complicated UTIs and pyelonephritis.
Table 4. Antimicrobial resistance rates among common uropathogens from patients hospitalised in the USA and Canada in 1997a
Q5. What is the role of the fluoroquinolones in the treatment of UTIs caused by P. aeruginosa, and is there any difference in activity against this pathogen among the individual fluoroquinolones?
A5. In regard to antibacterial activity, a 1996 study by Barry et al. compared the MICs of six different organisms and percent sensitivity for a range of fluoroquinolones (14). Results showed that levofloxacin and ciprofloxacin were similar except for the Enterococcus spp., which were more sensitive to levofloxacin (74% versus 61% for ciprofloxacin) and P. aeruginosa, which had an 82% sensitivity rate for levofloxacin compared to 87% for ciprofloxacin. Other fluoroquinolones did not appear to differ significantly from levofloxacin against P. aeruginosa. While levofloxacin has a lower in vitro potency against P. aeruginosa than ciprofloxacin, it has been shown to be effective in treating patients with these infections (15). This efficacy may be due to the pharmacokinetics of levofloxacin, as a 500 mg once-daily dose of levofloxacin produces a 4-fold higher AUC than ciprofloxacin 500 mg b.i.d. It has been shown by MacGowan et al. that an AUC/MIC ratio of 125 is predicted in 85.4% of levofloxacin administrations against P. aeruginosa, compared with 81.5% for ciprofloxacin (16). In my own personal clinical experience using 250 mg levofloxacin, I have had a 64% success rate in treating Pseudomonal infections (17), while Klimberg et al. achieved improved results, reporting a 85.75% success for levofloxacin compared to 66.7% for lomefloxacin (7). However, it must be noted that the number of Pseudomonal infections in these studies was small.
Q6. What investigations should be initiated when the patient with UTI is first seen?
A6. It is imperative to first get a good history and a complete physical, which will give you the information to decide which drug to use, and the length of treatment needed. I would ask for evidence of urgency, frequency, and dysuria, which are all lower UTI symptoms. In addition, the physician needs to check for suprapubic pain, which may be found in 10%, and bloody urine, whereas more complicated cases requiring a different treatment regimen will generally present with fever, severe back pain, vomiting, nausea, malaise, chills, possibly dehydration, and sepsis. It is important to check whether the patient is pregnant or breastfeeding, has allergies, their sexual and contraceptive history as well as previous UTIs. Finally, we need to know if the patient has any predisposing factors such as diabetes, or a history of stones or transplantation. This information will allow the clinician to differentiate the problem into a simple acute cystitis, acute pyelonephritis – either mild, moderate, or severe, or whether the patient has a complicated UTI. In addition to the history, we need to confirm the presence of bacteriuria or pyuria to make a diagnosis of UTI. Therefore, we take a clean catch mid stream urine (MSU) specimen, look for bacteriuria which can be done by performing a Gram stain. This simple procedure can be particularly valuable in young women who may have a S. saprophyticus infection. I do not order a urine culture in young women with uncomplicated acute cystitis, but this is required in all other patients to confirm the identification and sensitivity of the isolate. Additional requirements may be laboratory tests, including urinary creatinine, electrolytes and imaging studies, such as an ultrasound to check for obstruction. You may also need to perform a DMSA (dimercaptosuccinic acid) or DTPA (diethylenetriaminepentaacetic acid) scan in a few patients with complicated disease. All of these procedures are dependent on the initial history and examination, and are used to further differentiate the patients to assess whether they have complicated or uncomplicated infection, the severity of the disease, and the necessary duration of therapy.
Q7. Which UTIs do you think should be treated with levofloxacin? Could you comment on guidelines developed by professional societies for recommended treatment strategies in UTIs?
A7. I believe that the in vitro activity profile of levofloxacin coupled with an elimination half-life of seven hours with 100% bioavailability, excellent tissue penetration into the kidney, and high urinary penetration make it ideal for once-daily dosing in the treatment of UTIs. Of additional benefit is the fact that the very favorable pharmacokinetic and pharmacodynamic profile of levofloxacin, resulting in a high kidney concentration, is associated with a decreased tendency to select resistant organisms. The excellent efficacy of levofloxacin in treating UTIs has been recognized by the FDA, who have given broad approval for levofloxacin in acute cystitis, pyelonephritis and chronic bacteriuria.
In general, guidelines for treating UTIs are hard to establish because of the constant changing situation in which patients and clinicians find themselves. For instance, as already noted, the sensitivity profile of pathogens is changing, and the type of patients seen has also changed with an increase in those with AIDS and transplantation quite different to what it was 20 years previously. In the future, it is likely that these changes will continue, with developments in testing and diagnosis, as well as treatment options. Thus, while guidelines serve a purpose in helping provide a framework for the clinician, it must be remembered that they need to be constantly monitored and updated. I believe we always need to return to the individual patient to check to see how long therapy should be continued, and that guidelines need to be individually managed. Guidelines have been reported by Warren et al., which were commissioned by the Infectious Diseases Society of America and American Urological Association (18), as well as by Blondeau, aimed at helping physicians treat uncomplicated acute cystitis and acute pyelonephritis (19).
In acute cystitis, they recommend using either TMP-SMX b.i.d. for three days, or a fluoroquinolone for three days, but if the patient is in a high index resistance area, it is better to treat with a fluoroquinolone. If the patient has acute cystitis and diabetes, treatment should be continued for a longer duration, namely seven days. It is important to draw attention to the fact that (β-lactams are not highly recommended for acute cystitis, although nitrofurantoin is acceptable. Thus, in summary, TMP-SMX or a fluoroquinolone are recommended in acute cystitis, although my preference is levofloxacin as it can be given once daily.
Acute pyelonephritis requires a different treatment schedule. If the infection is severe, therapy should last for 14 days, with fluoroquinolones being the drugs of choice. Again, I would use levofloxacin because it is a once-daily agent that is available as IV for severely ill patients, with an easy switch to PO therapy after the patient has stabilized. The ease of switching from IV to PO levofloxacin is due to the extremely high oral bioavailability possessed by levofloxacin. In mild to moderate cases of acute pyelonephritis, the guidelines recommend seven days of therapy using a fluoroquinolone or TMP-SMX. However, I tend to use 5-7 days with the treatment duration usually empiric and dependent on how the patient is doing. For example, a very ill patient may require IV hydration for 18 hours, and once their fever has abated they may be able to return home on PO levofloxacin. Other patients with greater sepsis may need a longer course of therapy, and this highlights the need for flexibility in treatment durations.
Guidelines for treating complicated UTIs can be more difficult, due to the associated problems patients may have. For instance, you need to check the patient’s kidney function by assessing their glomerular filtration rate (GFR), because if this is decreased they may require a change in dosage. Complicated UTIs involve patients with other diseases, such as diabetes, renal transplants, lupus, and asthma, with the majority of these patients on other medications. It is very important in this situation to think of potential drug-drug interactions. Some of the most important of these include calcium antacids, digoxin, theophylline and cyclosporin. Levofloxacin stands out here as it has very few interactions, and does not interact with theophylline, digoxin, and other drugs which are metabolized in the cytochrome P450 system in the liver. In contrast, the other fluoroquinolones, which are metabolized by the liver, do have the potential for these interactions to occur. Thus, in complicated UTIs, I would hydrate the patient if necessary, give IV levofloxacin, then change to PO as soon as the patient’s infection has stabilized. Again, the duration of hospitalization and therapy will depend on the patient, and may be as little as 10 days increasing to 15 days if they are very ill.
Q8. Have you found in your own experience that you can get patients out of hospital earlier and reduce costs?
A8. Definitely. In fact, we need to consider the most effective management for reducing their length of stay as soon as the patient is admitted. Managed care protocols have been used to reduce the length of stay, and levofloxacin is very useful in this regard. Discharge is now determined by patient status, and levofloxacin therapy allows the patient to stabilize very quickly and be discharged on effective once-daily PO therapy. This is certainly associated with reduced costs in terms of hospitalization, which is a very expensive part of any treatment.
Q9. Could you summarize any randomized, comparative trials evaluating fluoroquinolones in UTIs.
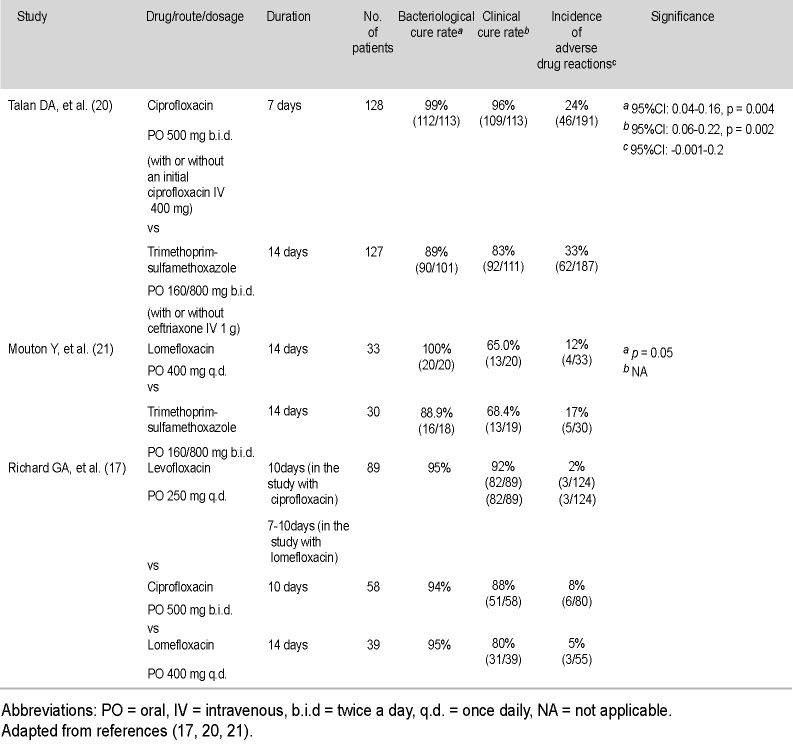
A9. The new fluoroquinolones have been proven to be very effective against both uncomplicated cystitis and acute pyelonephritis (Table 5) (17, 20, 21) in a number of randomized controlled trials. One 1998 trial investigated levofloxacin 250 mg versus ofloxacin 200 mg b.i.d. in uncomplicated cystitis, given for three days (22). The clinical success rate (cure plus improved) was 98.1% for levofloxacin and 97% for ofloxacin. The bacterial eradication rate was 96% for levofloxacin compared to 93% for ofloxacin.
Investigation into acute pyelonephritis has been performed by a number of researchers. I have conducted a trial investigating levofloxacin 250 mg PO once daily, versus ciprofloxacin 500 mg PO b.i.d. (17). The duration of ciprofloxacin therapy was 10 days, and only 7-10 days for levofloxacin. The bacteriological cure rate for levofloxacin was 95%, and clinical cure rate 92%, while respective figures for ciprofloxacin were 94% and 88%. A similar study was done comparing levofloxacin versus lomefloxacin 400 mg once daily, given for 14 days (17). The cure rate for lomefloxacin was lower at 80% compared to the 95% found for levofloxacin, although this was not statistically significant.
A study by Talan et al. looked at ciprofloxacin versus TMP-SMX, reporting a 96% success rate for ciprofloxacin compared to 83% for TMP-SMX (20). This is another example showing TMP-SMX is no longer as efficacious as it once was, and we already know levofloxacin is as successful as ciprofloxacin.
A study by Klimberg et al. investigating levofloxacin versus lomefloxacin in acute pyelonephritis demonstrated levofloxacin 250 mg once daily to be better than lomefloxacin 400 mg once a day (7). The clinical success rate for levofloxacin was 93.0% compared to 88.5% for lomefloxacin. Bacterial eradication was reported in 95.5% of isolates treated with levofloxacin, compared to 91.7% for the lomefloxacin treated group.
There has been one randomized trial in complicated UTIs, which I performed comparing levofloxacin versus ciprofloxacin (23). One hundred and twenty-six patients were enrolled in the levofloxacin arm and 113 in the ciprofloxacin arm. Clinical success was reported to be 92% for levofloxacin compared to 89% for ciprofloxacin. There was no significant difference in bacterial eradication, with a rate of 91% for the levofloxacin arm compared to 93% for the ciprofloxacin arm. However, within this study, a large difference was noted in effect on the confirmed Gram-positive organisms. For the 11 pathogens treated by levofloxacin, a 91% success rate was attained, while for the 12 Gram-positives in the ciprofloxacin arm, only a 50% eradication rate was achieved.
Table 5. Clinical results for the treatment of acute pyelonephritis among fluoroquinolones and other agents
Q10. Bringing together all of this trial data, what would you conclude about the clinical and bacterial efficacy of levofloxacin versus comparator agents in terms of microbiological and clinical outcome in UTIs?
A10. Summarizing these results, I believe it is fair to say overall that the comparative trials of once daily levofloxacin confirm it to be as efficacious as any current drug used in acute cystitis, acute pyelonephritis, and complicated UTI. It possesses broad-spectrum activity against Gram-negative and Gram-positive organisms, favorable pharmacokinetic and pharmacodynamic profiles and has been shown to have a decreased tendency to cause multiresistant emergence of pathogens, such as P. aeruginosa and Proteus spp. Several studies have proven it to be effective and safe against all classes of UTI, including acute uncomplicated cystitis, acute pyelonephritis, and complicated UTI. The once-daily dosing is very advantageous, as is its equivalent PO and IV bioavailability.
Q11. How does levofloxacin compare to comparator agents in terms of tolerability?
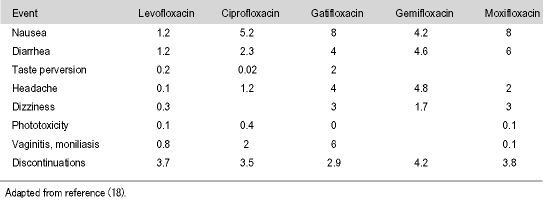
A11. Levofloxacin is associated with a low rate of adverse drug reactions (ADRs). In fact, it now has a huge post-marketing database of over 200 million prescriptions worldwide, confirming its exceptional safety in all patient populations. In the comparative trial I performed among levofloxacin, ciprofloxacin and lomefloxacin, the rate of ADRs were 2% for levofloxacin contrasted with 8% for ciprofloxacin and 5% for lomefloxacin (Table 5) (17). None of these were statistically significant. Nausea is the most common ADR seen with levofloxacin, although it is generally well tolerated, and this is at a much lower rate than that occurring with the other fluoroquinolones (Table 6) (18). In my own experience, we found levofloxacin to be very well tolerated. When treating UTIs, it is apparent that non-fluoroquinolones tend to have higher rates of ADRs than fluoroquinolones. One study comparing ciprofloxacin with amoxicillin-clavulanic acid found a minimal 2.7% rate for ciprofloxacin ADRs, compared to a much higher 21.2% for amoxicillin-clavulanic acid (24). I have also found from clinical experience that when using cephalosporins, amoxicillin, and ampicillin to treat UTIs, that many of the female patients develop Candida vaginitis, which in most cases is worse than their initial UTIs. These drugs are not as well tolerated, usually having a 15-20% incidence of ADRs reported. The great benefit with levofloxacin is that it is one of the least likely fluoroquinolones to cause any cardiovascular, central nervous system (CNS) effects or phototoxicity.
Table 6. Incidence (%) of fluoroquinolone-related adverse drug reactions
Q12. Do you use levofloxacin in preference to ciprofloxacin?
A12. Yes, I recommend it absolutely. Why give something twice daily, which is the case with ciprofloxacin, when you can use levofloxacin once daily. This is of particular benefit in those patients who may not be compliant. Many patients only take medications once daily and we need to be realistic about this.
Q13. What is your opinion or impression on differences between levofloxacin and gatifloxacin?
A13. Gatifloxacin is also renally excreted like levofloxacin and therefore bypasses hepatic metabolization. I have no comparative data between levofloxacin and gatifloxacin, but I have performed a study which investigated gatifloxacin 400 mg once daily for one day, 200 mg once daily for three days, 200 mg t.i.d. for one day, and ciprofloxacin 100 mg b.i.d. for three days. This was a large study with over 1,300 patients (25). The clinical and bacteriological responses were excellent at all levels. Gatifloxacin achieves very high blood concentrations, but the concentrations in the urine are not as high as those achieved by levofloxacin. An area where there is a potential for differences would be in safety and the rates of drug-drug interactions. At present, there is not enough data to support the safety and lack of interactions for gatifloxacin compared to the massive safety database for levofloxacin. In addition, it is known that gatifloxacin has the same CNS problems with non-steroidal anti-inflammatory drugs(NSAIDs) as some of the other fluoroquinolones.
Q14. Would you use different dosages/durations of levofloxacin therapy depending on the severity of UTIs and the site of infection?
A14. The treatment schedule depends on the response to therapy and the patient. In acute cystitis, a three-day treatment schedule is sufficient to achieve excellent results. In those with mild-to-moderate pyelonephritis, a 5-7-day course is used, while a patient with severe disease may require a more prolonged treatment schedule ranging from 7-14 days depending on the patient. The common factor is that all patients only require the treatment once daily. Even in patients who have had prior transplantation, or have AIDS, I still use a once-daily levofloxacin treatment. Pharmacological research looking at the Cmax to MIC(peak/MIC) ratio has confirmed that levofloxacin is effective when given in this way, and in addition, the postantibiotic effect associated with levofloxacin adds to the efficacy of the once-daily dose.
Q15. Do you have any experience with using a higher dose of levofloxacin?
A15. In my own practice, I have not used the higher doses that are available, as it is extremely effective in UTIs using 250 mg. However, I know that in other infections, such as those of the skin and soft tissues, 750 mg doses of levofloxacin have been used, while still maintaining its excellent safety profile.
Q16. Recurrent uncomplicated UTIs are a very common problem seen in the outpatient setting. Could you comment on the advantages/disadvantages of patient-initiated treatment in this setting? Would you recommend the use of levofloxacin in these patients?
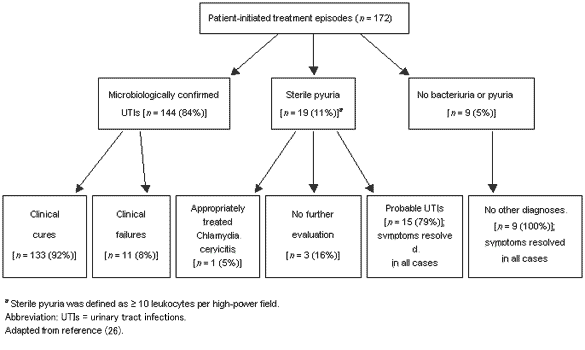
A16. A very good study has recently been reported on this issue, which investigated patient-initiated treatment of uncomplicated UTIs in young women (26). In the past, the usual approach to managing these patients was to use low-dose antimicrobial prophylaxis post-coitally, either daily or three times a week. Research has shown that this schedule is safe and effective. However, it can result in overuse of antibiotics as it is now recognized that recurrent episodes tend to occur in clusters, and if the antibiotic prophylaxis is used all of the time it results in unnecessary use in those with infrequent or clustered recurrences. This study by Gupta et al. enrolled female college students over 18 years of age, with a history of recurrent UTIs and no recent pregnancy, hypertension, diabetes or renal disease (Figure 1) (26). Subjects were educated about the problem, then given supplies of either 250 mg levofloxacin once daily for three days, or 200 mg ofloxacin b.i.d. for three days. Patients collected a MSU sample as soon as symptoms developed, then took the medication. The urine specimen was analyzed within 24 hr of collection. On days 10 and 30 after initiation of therapy, they returned to the clinic for follow-up urinalysis and interview, at which point they were given a new refill of medication and urine collection kit. One hundred and seventy-two women were enrolled and followed for 2-12 months each (mean of eight months). During the investigation, 88 of the 172 self-diagnosed at least one UTI. These women experienced 172 symptomatic events, and pre-therapy urinalysis confirmed the presence of a uropathogen in 84%, sterile pyuria in 11%, and no pyuria or bacteriuria in 5% (Figure 1) (26). Overall, 159 of 169 evaluable episodes, which resulted in self-treatment, were classed as UTIs needing therapy. Clinical cure was reported for 133 of 144 (92%) of the infections. It was also noted that all of the women with probable UTIs with no pyuria or bacteriuria improved following treatment. Some of those with negative cultures who improved may have had a Chlamydia or Ureaplasma urealyticum infection, or had the urethral syndrome defined as urethritis, cystitis, pyuria, and no bacteriuria, which occurs in 15-20% of young women (18-25 years) with no positive culture. The importance of this is now being recognized with a study underway investigating the role of U. urealyticum as an inducer of susceptibility to UTI in young women. In this year-long study, there were no reports of serious ADRs, and assessment of patient satisfaction showed the women to be very well satisfied, feeling they were able to start treatment earlier, with a shorter duration of symptoms, and able to resume work or school sooner than when they were managed traditionally.
There were many advantages identified by this study. We know that acute cystitis, although rare, can go on to develop into a complicated UTI, with early treatment stopping this progression. By initiating therapy immediately, the women were able to resume normal activities sooner, thereby cutting the cost considerably. It was a safe, very effective, convenient treatment. In addition, it was shown that women do not need to be on continuous prophylaxis, but only required episodic therapy. Eighty-four of those enrolled did not have a recurrent infection during the study and therefore did not need any treatment. Under standard therapy, they would have been on long-term prophylaxis, which would have been unnecessary in this study. While there were no major disadvantages noted, it was important that the subjects be carefully chosen to make sure they understand the practice to follow and are compliant. Levofloxacin works well here because it is an effective once-daily treatment.
Figure 1. Diagnostic accuracy and outcomes of 172 patient-initiated treatment episodes of recurrent urinary tract infection.
Q17. What is the role of parenteral therapy in treating complicated UTIs? Levofloxacin can be switched easily from IV to PO administration. What are the benefits of this switch therapy?
A17. There are some patients with severe infection and sepsis who require parenteral therapy. In these patients, we want to treat them as effectively as possible, give them hydration and then switch to oral therapy as soon as possible. This provides important cost benefits, with time in hospital lowered, and costs of parenteral therapy reduced. Levofloxacin is excellent in this regard as it is very easy to switch from IV to PO, administration, with no need to worry about dosage adjustments. This ease of use is very beneficial. In addition, it is nice to be able to use one drug, give it IV and switch to PO, because if there is an ADR you know it is due to the one agent. In contrast, if we use one drug IV and then switch to a different oral agent, it is impossible to confirm which agent causes the ADR. The fewer medications a patient gets the better, less chance of ADRs and better chance of identifying the problem.
Q18. Are there any subgroups of UTI patients that you feel would particularly benefit from levofloxacin treatment?
A18. It has the potential to be useful in patients with liver disease in whom other fluoroquinolones may not be indicated. Levofloxacin is also very useful in patient-initiated treatment because of its once-daily administration, and does not have any major drug-drug interactions, compared to some of the other fluoroquinolones. For instance, I would recommend levofloxacin rather than other agents when a patient is also taking warfarin, theophylline, or cyclosporin. The lack of interactions is very important because a lot of people are on asthmatic medications, digoxin, and cyclosporin as the transplant population is evergrowing.
Q19. What differences are there in terms of gender in regard to the incidence of different forms of UTI?
A19. Gender differences vary with age. In the pediatric setting, infants one year of age or less, the male/female ratio is equal and infections in this group occur mainly due to anatomical abnormalities. In the 1-5-year age group, reflux becomes an issue, and UTIs in this age group are more likely to occur in females (incidence in 1-5-year old girls is 4-5% compared to 0.5% or less in males). In the 6-16-year age group, it is again a female problem, occurring in 4-5% of girls and 0-0.5% of boys. Turning to the young adult population, 16-35 years, 20% of women develop UTIs, compared to only 0.5% of males. Therefore, until the age of 35 years, almost all patients with urological infections are females. After this time, there is a distinct change, with 35% of females and 20% of males developing UTIs in the 36-65-year old group. Beyond 65 years of age, the incidence tends to be high in both sexes, with many asymptomatic.
Q20. Do you see a role for fluoroquinolones in prophylaxis to avoid recurrent UTI?
A20. In the past, prophylaxis has been useful in select patient populations, but the results from Gupta et al.’s (26) patient-initiated study suggest a new approach which I like. However, this will not be practical for all patient groups, and prophylaxis will continue to be necessary in some people. In these cases, levofloxacin would work well. For example, in surgical cases, as well as in patients who have vesicoureteral reflux and those with recurrent UTIs. In general, prophylaxis is best given at night to counteract the logarithmic bacterial growth, so I would use 250 mg levofloxacin at night. While it has been argued that the fluoroquinolones should not be used in this way, solely in order to prevent the possible development of resistance, surveillance studies have not shown this to happen. Therefore, I think it is safe to use prophylaxis in the patients requiring it, such as those not suited to patient-initiated protocols.
![]()
- Stamm WE, McCue JD. Managing the challenge of UTI. Patient Care 1995; Feb: 1-17.
- Wimer S, Schoonover L, Garrison M. Levofloxacin: a therapeutic review. Clin Ther 1998; 20: 1049-70.
- Preston SL, Drusano GL, Bermon AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajjan J, Corrado M. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 1998; 279: 125-9.
- Langtry H, Lamb H. Levofloxacin. Its use in infections of the respiratory tract, skin, soft tissues and urinary tract. Drugs 1998; 56: 487-515.
- North D, Fish D, Redington J. Levofloxacin, a second generation fluoroquinolone. Pharmacotherapy 1998; 18: 915-35.
- Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother 2000; 16 (Suppl 1): 1-7; discussion 63-5.
- Klimberg IW, Cox CE, Fowler CL, King W, Kim S-S, Callery-D’Amico S. A controlled trial of levofloxacin and lomefloxacin in the treatment of complicated urinary tract infection. Urology 1998; 51: 610-5.
- Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med 2001; 135: 41-50.
- Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med 1993; 329: 1328-34.
- Jones RN, Kugler KC, Pfaller MA, Wusokur PL, and The SENTRY Surveillance Group, North America. Characteristics of pathogens causing urinary tract infections in hospitals: results from the SENTRY Antimicrobial Surveillance Program, 1997. Microbiol Infect Dis 1999; 33: 1-9.
- Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 1999; 281: 736-8.
- Stamm WE. Criteria for the diagnosis of urinary tract infection and for the assessment of therapeutic effectiveness. Infection 1992; 20 (Suppl 3): S151-4; discussion S160-1.
- Stamm WE, McKevitt M, Counts GW. Acute renal infection in women: treatment with trimethoprim-sulfamethoxazole or ampicillin for two or six weeks. A randomised trial. Ann Intern Med 1987; 106: 341-5.
- Barry AL, Fuchs PC, Brown SD. In vitro activities of five fluoroquinolone compounds against strains of Streptococcus pneumoniae with resistance to other antimicrobial agents. Antimicrob Agents Chemother 1996; 40: 2431-3.
- Martin SJ, Jun P, Garvin CG. A risk benefit assessment of levofloxacin in respiratory, skin and skin structure, and urinary tract infections. Drug Saf 2001; 24: 199-222.
- MacGowan AP, Wootton M, Holt HA. The antibacterial efficacy of levofloxacin and ciprofloxacin against Pseudomonas aeruginosa assessed by combining antibiotic exposure and bacterial susceptibility. J Antimicrob Chemother 1999; 43; 345-9.
- Richard GA, Klimberg IN, Fowler CL, Callery-D’Amico S, Kim S-S. Levofloxacin versus ciprofloxacin versus lomefloxacin in acute pyelonephritis. Urology 1998; 52: 51-5.
- Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999; 29: 745-58.
- Blondeau JM. Clinical utility of the new fluoroquinolones for treating respiratory and urinary tract infections. Expert Opin Investig Drugs 2001; 10: 213-37.
- Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Ruening-Scherer J, Church DA. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women. A randomised trial. JAMA 2000; 283: 1583-90.
- Mouton Y, Ajana F, Chidiac C, Capron MH, Home P, Masquelier AM. A multicenter study of lomefloxacin and trimethoprim/sulfamethoxazole in the treatment of uncomplicated acute pyelonephritis. Am J Med 1992; 92 (Suppl 4A): S87-90.
- Richard GA, DeAbate CA, Ruoff GE, Corrado M, Fowler CL, Morgan N. A double-blind, randomized trial of the efficacy and safety of short-course, once-daily levofloxacin versus ofloxacin in uncomplicated urinary tract infections. Infect Dis Clin Pract 1998; 9: 323-9.
- Richard GA, Childs SJ, Fowler CL, Pittman W, Nicolle LE, Callery D’Amico S. Safty and efficacy of levofloxacin in complicated urinary tract infections in adults. Pham Ther 1998; 23: 534-40.
- Lipsky BA, Baker CA. Fluoroquinolone toxixity profiles: a review focusing on newer agents. Clin Infect Dis 1999; 28: 352-64.
- Richard GA, Chavaramplakic PM, Kristein JM, Orchard D, Yang JY. Single-dose fluoroquinolone therapy of acute uncomplicated urinary tract infections in women: results from a randomized, double-blind trial comparing single-dose to three-day fluoroquinolone regimens. Urology 2002 (in press).
- Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med 2001; 135: 9-16.










