Levofloxacin -- The "Respiratory Fluoroquinolone"

Carl A. DeAbate , MD
Medical Director
Medical Research Center
New Orleans, LA
USA
Levofloxacin, the active l -isomer of ofloxacin, has all of the excellent features of its parent compound, and the additional advantages of being even more effective against a wider spectrum of bacteria at half the dosage, making it exceedingly well tolerated. These characteristics have rapidly proven levofloxacin to be one of the most promising of the new fluoroquinolones. Levofloxacin’s broad spectrum of activity encompasses most of the common pathogens of the respiratory tract, and its pharmacokinetic features allow it to penetrate extremely well into lung tissue and bronchial secretions. Such attributes have earned levofloxacin the title of a “respiratory” quinolone, and its efficacy in a once-daily dosing schedule and good safety profile, have made it well accepted by most patients.
To characterise the role of levofloxacin in the treatment of acute bacterial exacerbations of chronic bronchitis (ABECB), one of the respiratory tracts most ubiquitous infections, Penetrationinterviewed Dr. Carl A. DeAbate, Medical Director, Medical Research Center, New Orleans, USA. He has recently performed a clinical study comparing levofloxacin versus cefuroxime axetil in the treatment of adult outpatients with ABECB. Dr. DeAbate was able to draw on his wide experience in treating respiratory tract infections (RTI) to discuss the role of levofloxacin in the therapy of these common infections.
![]()
Questions
Q2. What advantages does this pharmacokinetic profile give levofloxacin over other fluoroquinolones?
Q3. What pharmacodynamic advantages does levofloxacin provide in the treatment of ABECB?
Q8. Would you use levofloxacin in combination with other antibiotics in ABECB?
Q10. How did you assess efficacy and safety of treatment?
Q11. Could you describe the clinical results of your trial investigating levofloxacin in ABECB?
Q12. How did the microbiological response compare between levofloxacin and cefuroxime axetil?
Q13. Could you comment on the safety profile of levofloxacin compared to other agents?
![]()
Answers
Q1. Could you comment on the pharmacokinetic features of levofloxacin that make it a useful drug in acute bacterial exacerbations of chronic bronchitis (ABECB)?
A1. Levofloxacin possesses the excellent pharmacokinetic properties of ofloxacin, its parent compound. In addition, it has two to four times the potency of ofloxacin in vitro , and demonstrates exceptional activity against most aerobic Gram-positive and Gram-negative organisms associated with respiratory tract infections (RTI). There are several other properties of levofloxacin that contribute to its usefulness. It is rapidly absorbed, is extensively distributed into the tissues, and concentrations of the drug are typically found to be higher in the tissues or body fluids than in plasma. Peak plasma concentrations are seen in less than two hours and are dose-dependent (1) . With approximately 90% of the dose excreted in the urine, a half-life of six to seven hours, and a post-antibiotic effect of two to three hours, levofloxacin can be used as a very effective once-daily therapy, which offers convenience and increased compliance for ABECB patients.
Q2. What advantages does this pharmacokinetic profile give levofloxacin over other fluoroquinolones?
A2. Several issues come to mind when we compare levofloxacin to other fluoroquinolones, and perhaps the most important is the problem we have encountered with resistance. Levofloxacin has two important advantages over many other fluoroquinolones in this regard. Firstly, it uses two mechanisms of action for bactericidal activity, one requiring RNA and protein synthesis, and another which does not. Most of the other fluoroquinolones use only one method, so the risk of developing resistance to them is higher. The second advantage with levofloxacin is its use as a single once-daily dose, which significantly reduces the risk of developing resistance compared with other fluoroquinolones which must be taken more frequently. Of course, the fact that it demonstrates 100% bioavailability, has a broad spectrum of activity, and produces comparatively fewer side effects also contribute to its favorability over other fluoroquinolones.
Q3. What pharmacodynamic advantages does levofloxacin provide in the treatment of ABECB?
A3. The observation that levofloxacin penetrates well into bronchiolar tissue and sputum is significant when treating patients with RTI. The sputum concentration of the drug following oral administration has been reported to range from approximately 85% to 95% of serum concentrations (2) . These findings suggest that clinically effective concentrations of levofloxacin are achieved at target respiratory sites, exceeding the minimum inhibitory concentrations (MIC) for common respiratory pathogens. It also exhibits greater activity against streptococci than both ciprofloxacin and ofloxacin and a greater potency against many anaerobes and Gram-positive organisms.
Q4. What are the usual pathogens involved in ABECB and how does this relate to the antibacterial spectrum of levofloxacin? How do the MIC values compare to other available treatments?
A4. Typical pathogens in ABECB include organisms such as Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, and Moraxella catarrhalis, all of which are susceptible to levofloxacin. Previous studies have shown that the MIC90 values are approximately 50% lower against Gram-positive and Gram-negative organisms than ofloxacin and three to four times lower than ciprofloxacin for methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) (3) .
Q5. What is the role of atypical pathogens in ABECB and how effective is levofloxacin compared to other commonly used agents against these bacteria?
A5. Atypical pathogens such as Mycoplasma pneumoniae,Chlamydia pneumoniae, and Legionella pneumophila are important to identify as disease-causing agents because they can contribute to superinfections, so a drug that can eradicate both the typical and atypical pathogens at the same time is desirable. Levofloxacin has shown excellent activity against these organisms and is as effective as other standard therapies. With resistance to traditional therapies becoming such as issue, it is important that we can identify a medication such as levofloxacin which offers both potency and safety with a shorter dosing time.
Q6. Could you comment on any other studies comparing levofloxacin to other antimicrobials (for example cefaclor) in the treatment of ABECB?
A6. Results from a recent trial comparing oral levofloxacin 500 mg daily to cefaclor 250 mg three times daily in patients with ABECB found that among clinically evaluable patients treated with levofloxacin, 72.1% were cured and 19.5% improved compared with 64.5% cured and 27.1% improved with cefaclor. The microbiologic eradication rates by pathogen were 95% for levofloxacin and 86.5% for cefaclor. There was 100% eradication of H. influenzae, 94.7% eradication of M. catarrhalis, and 90% eradication of S. pneumoniae for levofloxacin, compared to eradication rates of 70.8%, 100% and 85.7%, respectively, for the cefaclor group. The findings indicate that levofloxacin given for five to seven days was as effective as cefaclor given for seven to ten days for ABECB (4) . Interestingly the results of this study were similar to results obtained from our study comparing levofloxacin and cefuroxime axetil.
Q7. With the oral form of levofloxacin possessing excellent pharmacodynamic properties could it be used in place of alternative intravenous forms of therapy?
A7. Oral therapy with levofloxacin could replace intravenous (IV) therapy in most cases, unless the patient is incapacitated in some way in which it would be more practical to use an IV method. Peak plasma concentrations (Cmax) are very similar and the oral form is both less expensive and easier to administer than IV therapy.
Q8. Would you use levofloxacin in combination with other antibiotics in ABECB?
A8. I think combination therapy with levofloxacin could be very advantageous in the treatment of immunocompromised or elderly patients, particularly where resistance is an issue. We see resistance to staphylococci everyday, and the use of both levofloxacin and rifampin would likely be an effective method of treatment. The key is to use a combination of drugs which act in time-dependent and concentration-dependent killing manners, such as a ß-lactam with levofloxacin. In this scenario, we achieve a wide range of activity by utilising the different killing mechanisms of both classes, which provides us with greater potential for treatment and cure.
Q9. You have performed a comparative study to evaluate levofloxacin in the treatment of ABECB? Could you discuss this?
A9. Our study was designed to compare the clinical efficacy and tolerability of levofloxacin with cefuroxime axetil in the treatment of adult outpatients with ABECB. Cefuroxime axetil is a widely used broad-spectrum ß-lactam antibiotic often selected as primary empiric therapy for these infections because of its broad spectrum of activity. The study was an open-label trial in which patients who met entry criteria were randomly assigned to receive either one 500 mg tablet of levofloxacin orally once-daily for five to seven days or one 250 mg tablet of cefuroxime axetil orally twice-daily for ten days. The patients were adults who were experiencing an episode of ABECB and who could receive oral medication. The diagnosis of ABECB was based on a history of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema; a recent increase in cough; a change in the quality of sputum; and physical findings consistent with ABECB. Patients who had received prior antibiotic therapy could be enrolled if the therapy had lasted less than 24 hours or if there had been no stabilisation or improvement by prior therapy. Patients with acute bronchitis, pneumonia, cystic fibrosis or any other disease interfering with evaluation of either study drug were excluded. A thoracic radiograph was performed and Gram stain and culture were performed on sputum collected within 48 hours before admission. Respiratory specimens also included deep expectorated or suctioned sputum, transtracheal aspirates, or bronchial brushings, biopsies, or washings. Patients returned for a scheduled visit and evaluation of clinical progress on days three to five of study drug administration. Those patients with negative culture results on admission were allowed to continue in the study. Those with pathogens resistant to the study drug were allowed to remain in the study if there had been reasonable clinical improvement during the study. A post-therapy visit was scheduled for five to seven days after termination of therapy at which time a physical examination and repeat laboratory testing were performed.
Q10. How did you assess efficacy and safety of treatment?
A10. We assessed efficacy based on clinical signs and symptoms, clinical response rates, and microbiological eradication rates. Clinical symptoms of ABECB included chills, chest pain, shortness of breath, increased cough, increased sputum, and purulent sputum, which were graded as present or absent. Clinical signs included diminished breath sounds, rales, egophony, rhonchi, and wheezes. These were graded as none, mild, moderate or severe. At the post-therapy visit the clinical response was evaluated as cured, improved, failed or unable to evaluate. A clinical cure was defined as a resolution of the signs and symptoms; a clinical improvement was defined as an incomplete resolution of signs or symptoms with no further need for antimicrobial therapy; and a lack of response to therapy was deemed a clinical failure. If the patient could produce sputum, Gram stain, culture and sensitivity testing were carried out to assess microbiological response, which was assessed as eradicated, persisting or unknown. Susceptibility testing was performed for levofloxacin and cefuroxime axetil on pathogens isolated at admission and at the post-therapy visit if possible. For safety evaluations, we assessed the incidence of treatment-emergent adverse events; results of laboratory testing; and physical examination findings including vital sign measurements.
Q11. Could you describe the clinical results of your trial investigating levofloxacin in ABECB?
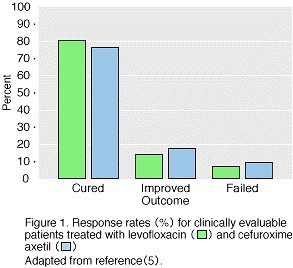
A11. This study was conducted at 34 centres and included 492 patients, of whom 248 received levofloxacin and 244 received cefuroxime axetil. The demographic characteristics and disposition of patients in the two study groups are outlined in Tables 1, 2 (5) . The clinical response was rated a success, with the patient cured or improved in 94.6% of those receiving levofloxacin and 92.6% of the cefuroxime axetil group (Table 3, Figure 1) (5) . All of the symptoms of bronchitis were resolved in more than 86% of the patients, with the exception of shortness of breath, which resolved in 69.7% of levofloxacin-treated and 72.4% of cefuroxime axetil-treated patients. Resolution or improvement of abnormalities on thoracic auscultation were noted in 85% or more of patients, with the exception of diminished breath sounds, which were resolved or improved in 62.5% of levofloxacin-treated and 55.2% of cefuroxime axetil-treated patients.
Table 1. Disposition of patients entered into antibiotic comparison study
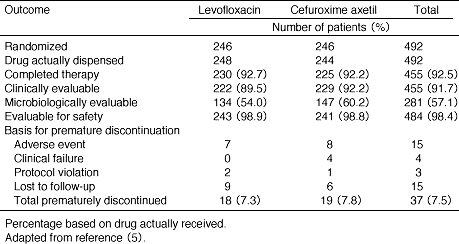
Table 2. Demographic and baseline characteristics of the modified intent-to-treata patient population
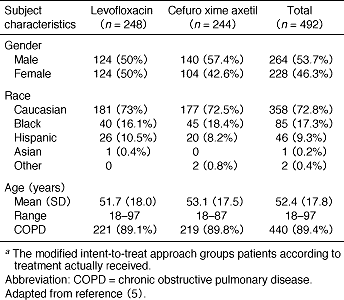
Table 3. Clinical response rates for patients with pathogens of primary interest (clinically evaluable patients)
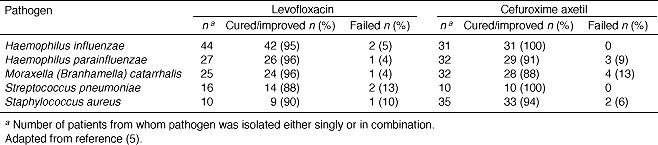
Figure 1.
Q12. How did the microbiological response compare between levofloxacin and cefuroxime axetil?
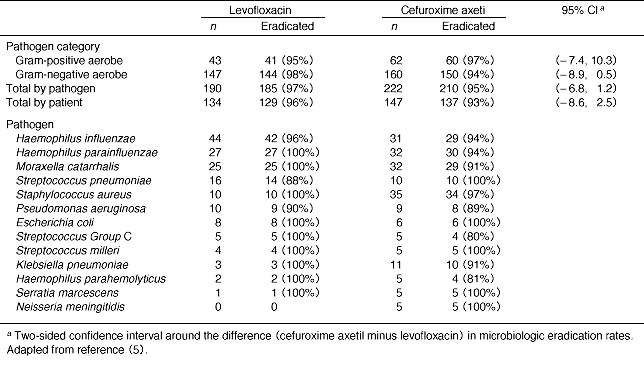
A12. Microbiological eradication rates for levofloxacin were impressive overall (Table 4) (5) . We found that the response rates were similar, but levofloxacin had a higher success rate overall. The microbiologic eradication rates by pathogen were 97.4% in the levofloxacin-treated group and 94.6% in the cefuroxime axetil-treated group, while the eradication rates by patient were 96.3% and 93.2%, respectively. We observed nearly twice as much microbiologic persistence in the cefuroxime axetil treated patients (6.8%) as with the levofloxacin-treated patients (3.7%). The drugs were comparable for both clinical and microbiologic response, although levofloxacin had the advantage of requiring fewer dosing days. Patients on levofloxacin required an average of seven dosing days, while the cefuroxime axetil-treated group was treated for ten days. This indicates that a significant clinical response can occur with a shorter treatment regimen of levofloxacin than cefuroxime axetil.
Table 4. Microbiological eradication rates summarized by pathogen category and pathogen for microbiologically evaluable patients
Q13. Could you comment on the safety profile of levofloxacin compared to other agents?
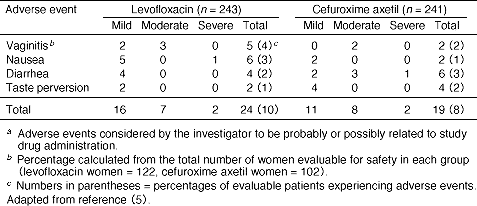
A13. In our study we experienced relatively few adverse drug reactions (ADR) associated with levofloxacin (Table 5) (5) . Of 243 patients taking levofloxacin, there were six reported cases of nausea, four reports of diarrhea, and two reports of taste perversion. In addition, five women reported mild or moderate vaginitis. Adverse events for cefuroxime axetil were comparable. Similar ADR have been noted for other quinolones but because levofloxacin is given at a lower dosage, we would expect even fewer ADR. I think levofloxacin is better tolerated than macrolides, as gastrointestinal discomfort was comparatively less than typically seen with macrolide therapy.
Table 5. Incidence of drug-related advers eventsa reported by 1% or more of patients evaluable for safety
Q14. Could you comment on the once-daily dosage schedule of levofloxacin you used, and benefits this provides?
A14. Results from our study indicate that levofloxacin is highly effective in the treatment of ABECB with a shorter treatment period than cefuroxime axetil. We found that a significant clinical response occurred in patients who took levofloxacin once-daily for an average of seven days, compared to twice-daily cefuroxime axetil for ten days. The ease of use of levofloxacin combined with comparable clinical results may enhance patient compliance beyond those observed with traditional empiric antibiotic therapies. The use of levofloxacin offers a shorter course of therapy, which means a less expensive prescription. This cheaper form of therapy would is likely to further increase compliance, which we would expect to be above average anyway when levofloxacin’s other advantages are considered.
Q15. Is resistance to treatment a problem in this setting, and how does it impact on your choice of treatment?
A15. Traditional antibiotics such as penicillin, ampicillin, and amoxicillin are exhibiting diminishing clinical and microbiologic activity against many of the most common respiratory pathogens associated with ABECB. The growing incidence of ß-lactam and macrolide resistance in these pathogens further complicates the selection of an effective form of therapy. Most physicians treat ABECB empirically and must consider several factors before choosing a treatment. These include an antibiotics spectrum of activity, the pathogens most likely to cause the infection, the local pattern of bacterial resistance and the overall empiric effectiveness of the antibiotic against pathogens associated with ABECB. The fact that most bacteria are susceptible to levofloxacin in addition to its ease of use make it a favorable form of therapy.
Q16. Is ß-lactamase production by pathogens becoming a major problem, and could you comment specifically on the development of multidrug-resistant pneumococcus, and how this affects treatment?
A16. ß-lactamase production by pathogens has been a problem for some time now and is becoming worse. We can only assume that the development of multidrug resistant pneumococcus will become much greater in the future, which will further limit our ability to effectively treat ABECB. To combat this resistance, combination therapies, higher doses of traditional therapies, or alternative therapies must be utilised.
Q17. It has been shown that in vitro MIC values do not always correspond to actual in vivo activity. Could you comment on this with reference to levofloxacin?
A17. I think it is possible that levofloxacin exhibits greater in vivoactivity against certain pathogens because it is found in such high levels in the serum and it is distributed so widely in the tissues. The fact that some in vitro MIC values do not reflect in vivoactivity could be attributed to the dosing schedule. When susceptibility is noted in vivo but not in vitro, it could be that higher doses may have been used to treat the infection than those used when MIC values were noted; thus, we would observe the pathogen overcoming resistance.
Q18. From the results of your clinical trial, and practical experience using this drug, could you comment on other RTI you would recommend levofloxacin be used for?
A18. We have seen outstanding results when using levofloxacin to treat ABECB. I would use it for any RTI due to similar pathogens, such as sinusitis, pharyngitis, and pneumonia, including atypical or community-acquired pneumonia.
Q19. What are the areas of future research that you think need to be carried out regarding the use of fluoroquinolones, in particular levofloxacin, in different RTI?
A19. Because levofloxacin has been proven so useful in the treatment of ABECB it would be very beneficial to conduct similar trials for sinusitis, pharyngitis and pneumonia. I would like to see how well a higher dose of levofloxacin (or any fluoroquinolone) could be tolerated. I would also welcome a study which paired levofloxacin with other antibiotics, to show which combinations work best.
![]()
-
- Davis R, Bryson HM. Levofloxacin: a review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs 1994; 47: 677-700. See the published erratum Drugs 1994; 48: 132.
- Nakamori Y, Tsuboi E, Narui K, Nakatani T, Nakata K, Sugi H. Sputum penetration of levofloxcain and its clinical efficacy in patients with chronic lower respiratory tract infections. Jpn J Antibiot 1992; 45: 548-56.
- Fu KP, Lafredo SC, Foleno B, Isaacson DM, Barrett JF, Tobia AJ, Rosenthale ME. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother 1992; 36: 860-6. See the published erratum Antimicrob Agents Chemother 1992; 36: 1797.
- Habib MP, Gentry LO, Rodriguez-Gomez G, Morowitz W, Polak E, Rae JK, et al. A multicenter, randomized study comparing the efficacy and safety of oral levofloxacin vs cefaclor in the treatment of acute bacterial exacerbations of chronic bronchitis. Proceedings of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. New Orleans, 1996.
- DeAbate CA, Russell M, McElvaine P, Faris H, Upchurch J, Fowler CL, et al. Safety and efficacy of oral levofloxacin versus cefuroxime axetil in acute bacterial excerbation of chronic bronchitis. Respir Care 1997; 42: 206-13.










