Changes and Updates in the New ATS/IDSA 2019 CAP Guidelines
Daiichi Sankyo’s ‘Meet the Expert Online’ webinar, held on 17 March 2021 and presented by Professor Lionel Ari Mandell of McMaster University, Canada, was convened to discuss new updates in the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) 2019 community-acquired pneumonia (CAP) guidelines.
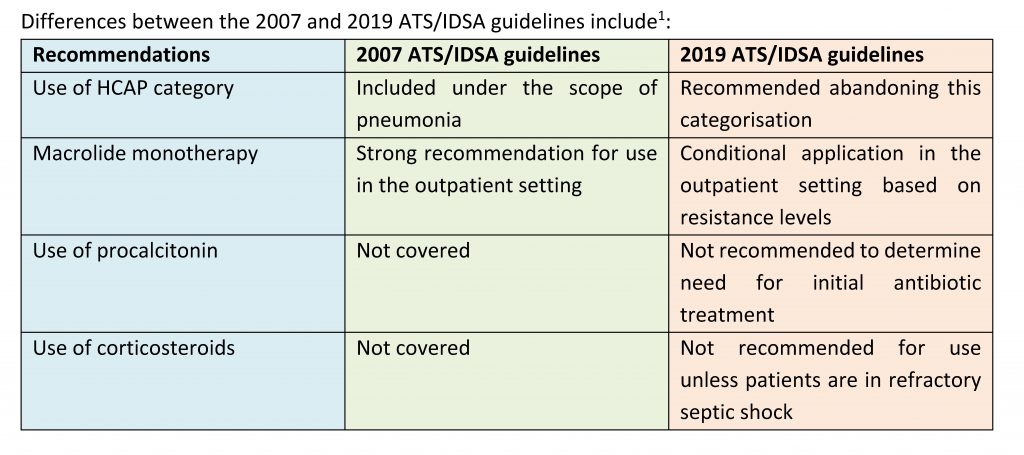
Pneumonia – change in scope
- The 2007 ATS/IDSA guidelines included CAP, healthcare-associated pneumonia (HCAP), and hospital- and ventilator-associated pneumonia (HAP/VAP) under the scope of pneumonia1
- Under the new 2019 ATS/IDSA guidelines, HCAP has been removed; pneumonia now covers CAP and HAP/VAP, with emphasis on local epidemiology and validated risk factors to determine need for methicillin-resistant Staphylococcus aureus(MRSA) or Pseudomonas aeruginosa coverage1
Pneumonia – change in treatment guidelines
The new 2019 ATS/IDSA guidelines reaffirm many recommendations from the guidelines published in 2007, with several changes made1:
- The implementation of a narrower scope (time of diagnosis, to the end of treatment) with less focus on epidemiology or pathogenesis1
- The use of the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) format to evaluate the Patient or Population, Intervention, Comparison, and Outcome (PICO) framework1
PICO question number 5: In Adults with CAP, should serum procalcitonin plus clinical judgement versus clinical judgement alone be used to withhold initiation of antibiotic treatment?
Recommendation1:
- In deciding whether to stop treatment, empiric antibiotic therapy should be initiated in adults with clinically suspected and radiographically confirmed CAP, regardless of initial serum procalcitonin level1
PICO question number 8: In the outpatient setting, which antibiotics are recommended for empiric treatment of CAP in adults?
Recommendations1:
- While few major clinical outcomes (mortality and treatment failure leading to hospitalisation) were reported, it is important to note that only a small sample size was used in these studies1
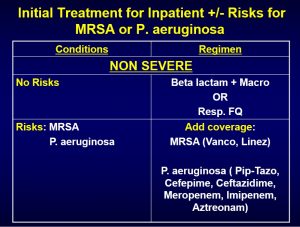
PICO question number 9: In the inpatient setting, which antibiotic regimens are recommended for empiric treatment of CAP in adults without risk factors for MRSA and P. aeruginosa?
In patients with non-severe conditions and not at risk of MRSA or P. aeruginosa, the following empiric treatment regimens are recommended1:
- A combination of beta-lactam and macrolide, or monotherapy with a respiratory fluoroquinolone (FQ) can be used1
In patients with severe conditions and not at risk of MRSA or P. aeruginosa, the following empiric treatment regimen is recommended1:
- A combination of beta-lactam and macrolide, or beta-lactam and a respiratory fluroquinolone can be used1
Key considerations1:
- More well-designed clinical trials are required1
PICO question number 10: In the inpatient setting, should patients with suspected aspiration pneumonia (AP) receive additional anaerobic coverage beyond standard empiric treatments for CAP?
Recommendation1:
- Routine anaerobic coverage for suspected AP should not be added unless lung abscess or empyema is suspected1
PICO question number 11: In the inpatient setting, should adults with CAP and risk factors for MRSA or P. aeruginosa be treated with extended-spectrum antibiotic therapy instead of standard CAP regimens?
Recommendations1:
- The use of prior categorisation of HCAP to guide selection of extended antibiotic coverage in adults with CAP should be abandoned1
- Both parenteral and/or oral antibiotics are risk factors; the guidelines only mention parenteral antibiotics
- Double agent coverage for aeruginosa should be started with a transition to single agent treatment if the patient is improving
Summary of recommended empirical treatment of patients with risks factors for MRSA or P. aeruginosa1:

Key considerations1:
- Important to distinguish between severe and non-severe cases
- Risk factors for MRSA or aeruginosa are often weakly associated
- The strongest risk is prior respiratory isolation
PICO question number 12: In the inpatient setting, should adults with CAP be treated with corticosteroids?
Recommendation1:
- The routine use of corticosteroids in adults with non-severe CAP, severe CAP and severe influenza pneumonia should not be used, unless in the case of refractory septic shock1
PICO question numbers 13: In adults with CAP who test positive for influenza, should the treatment regimen include antiviral therapy?
Recommendation1:
- Antivirals should be prescribed for adults with CAP who test positive for influenza in the inpatient setting, independent of duration of illness before diagnosis1
PICO question numbers 14: In adults with CAP who test positive for influenza, should the treatment regimen include antibacterial therapy?
Recommendation1:
- Standard antibacterial treatment should be initially prescribed for adults with clinical and radiographic evidence of CAP who test positive for influenza in the inpatient and outpatient settings1
PICO question number 15: In outpatient and inpatient adults with CAP who are improving, what is the appropriate duration of antibiotic treatment?
Recommendations1:
- The duration of antibiotic therapy should be guided by a validated measure of clinical stability, and antibiotic therapy should be continued until the patient achieves stability and for no less than a total of 5 days1
- Depending on the presence of comorbidities, complications and type of pathogens, treatment for 10–14 days may be required
PICO question number 16: In adults with CAP who are improving, should follow-up chest imaging be obtained?
Recommendation1:
- In adults with CAP whose symptoms have resolved within 5–7 days, routinely obtaining follow-up chest imaging is unnecessary1
Treatment principles in 2019 ATS/IDSA guidelines
- Macrolide monotherapy may be used in outpatients with mild CAP and preferably no comorbidity if the pneumococcal susceptibility to macrolides is 75% or higher
- The use of FQ monotherapy in outpatients and non-severe inpatients is supported, as it allows for (PICO question number 8)1:
- Very low resistance with CAP pathogens
- Coverage of typical and atypical CAP pathogens
- Overall bioavailability
- Convenient monotherapy use
- Clinical performance with both inpatients and outpatients
- Relative rarity of severe adverse effects related to use
- Beta-lactam should not be used for inpatient monotherapy
- Routine coverage of anaerobes for AP is unnecessary; steroids for severe CAP or influenza should only be used if there is refractory septic shock
- If both CAP and influenza are present, both antivirals and antibiotics should be administered
Summary of recommended treatment of CAP during the COVID-19 pandemic:
- If patient has CAP, but is negative for COVID-19: routine outpatient or inpatient treatment for CAP is implemented
- If patient has CAP, with pending results for COVID-19: routine treatment for CAP should be started and regimen will depend upon whether patient is hospitalised or not; COVID-19 treatment as per algorithm
- If patient has CAP and is positive for COVID-19: treatment for CAP should be started and regimen will depend upon whether patient is hospitalised or not; COVID-19 treatment as per algorithm
Levofloxacin
- The antibiotic levels in the epithelial lining fluid correlate with outcomes in pneumonia2,3
- Single agent 750 mg levofloxacin therapy is clinically and microbiologically as efficient and safe for the treatment of CAP as the existing standard of ceftriaxone and macrolide combination treatment4
- Levofloxacin is a valuable antimicrobial agent and an excellent treatment option for acute exacerbation chronic bronchitis, CAP and HAP5
- Benefits of levofloxacin include:
- The availability of levofloxacin in oral and intravenous forms
- Excellent bioavailability
- High concentration in the lung compartment
- Clinical efficacy and safety
References
- Metlay JP, et al. Am J Respir Crit Care Med Vol 2019;200:e45-e67.
- Gotfried MH, et al. Chest 2001;119:1114-22.
- Capitano B, et al. Chest 2004;125:965-73.
- Lee JH, et al. Clin Drug Investig 2012;32:569-76.
- Torres A, Liapikou A. Expert Opin Pharmacother 2012;13:1203-12.










