New Treatment Strategies for the Eradication of Helicobacter pylori—Combating the Global Trend of Antibiotic Resistance
Helicobacter pylori antibiotic resistance and eradication therapies—selected posters and oral presentations at the Gastro 2013 APDW/WCOG
Vilaichone RK, et al. Nationwide survey of antibiotic-resistant strains of H. pylori in Thailand.
Poster P0207.
- A total of 3,964 dyspeptic patients who underwent upper endoscopy from the North, Northeastern, Central and Southern regions of Thailand between January 2005 and December 2012 were enrolled
- 34.1% of patients were positive for H. pylori. E-tests for amoxicillin, clarithromycin, metronidazole and tetracycline were positive in 400 isolates, and for levofloxacin and ciprofloxacin in 208 isolates
- Resistance to antibiotics was present in 50.3% of patients, including to amoxicillin (5.2%), clarithromycin (3.7%), metronidazole (36%), tetracycline (1.7%), levofloxacin (7.2%) and ciprofloxacin (7.7%)
- Metronidazole resistance was significantly higher in Southern Thailand than in the Northeastern region (66.7% vs 33.3%; p=0.04)
- Clarithromycin resistance was significantly higher in patients aged older than 40 years (4.7% vs 0%; p=0.04)
- The authors concluded that metronidazole resistance remains the most common type of antibiotic resistance among H. pyloristrains in Thailand. In addition, age over 40 years is a predictor for clarithromycin resistance in Thailand
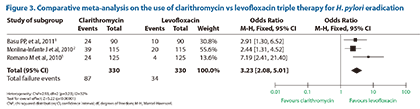
Rolluqui GF, et al. A comparative meta-analysis on the use of clarithromycin vs levofloxacin for H. pylori eradication.
Poster P0188.
- This was a study to evaluate the cure rates of triple therapy containing either clarithromycin or levofloxacin; it aimed to compare the effectiveness between the combination of clarithromycin, omeprazole and amoxicillin and the combination of levofloxacin, omeprazole and amoxicillin in the eradication of H. pylori
- The study was a comparative meta-analysis and involved a systematic search of randomized control trials using either clarithromycin or levofloxacin as part of the triple therapy for the eradication of H. pylori
- The meta-analysis involved 3 studies that included a total of 330 patients in each group
- The meta-analysis revealed levofloxacin-based therapies resulted in significantly lower eradication failure rates compared with clarithromycin-based therapies (34 vs 87 total failure events; p<0.00001; Figure 3)
- The authors concluded that a triple therapy consisting of levofloxacin, omeprazole and amoxicillin may be considered a better first-line treatment option for H. pylori eradication compared with the combination of clarithromycin, omeprazole and amoxicillin
 |
| Click on image to enlarge. |
References
- Basu PP, et al. Am J Gastroenterol 2011;106):1970-1975.
- Molina-Infante J, et al. Aliment Pharmacol Ther 2010;31:1077-1084.
- Romano M, et al. Gut 2010;59:1465-1470.
Pittayanon R, et al. Efficacy of dual therapy with high dose PPI and amoxicillin in H. pylori eradication.
Poster P0798.
- This was a study to determine the efficacy of dual therapy, consisting of rabeprazole 20 mg qid and amoxicillin 1 g tid, as first-line H. pylori eradication treatment
- A total of 72 patients with H. pylori associated gastritis were recruited. Patients were assessed for CYP2C19 genotype, as this determines PPI metabolizing activity
- The eradication success rate as determined by negative UBT was 72.2% (52/72 patients) and there was no reported drop out
Of the 50 patients who had CYP2C19 genotype identified, 40% were rapid metabolizers, 46% were intermediate metabolizers and 14% were poor metabolizers - The authors concluded that dual therapy with rabeprazole and amoxicillin is a simple, safe and effective regimen with minimal side effects. The efficacy of this regimen was independent of CYP2C19 genotype
El-Omar E. Will eradication of H. pylori actually prevent gastric cancer?
Session 3401.
Gastric cancer is the second leading cause of cancer death worldwide, with an estimated 990,000 new cases and 738,000 deaths in 2008.1 In 1994, the International Agency for Research on Cancer classified H. pylori as a carcinogen in humans. Since then, it has become increasingly accepted that colonization of the stomach with H. pyloriis an important cause of gastric cancer. Although eradication of the infection in early life should hypothetically reduce the incidence of this fatal tumor, it is still controversial whether H. pylori eradication therapy is effective in primary prevention of gastric cancer.
The Shandong Intervention Trial examined the 15-year effects of H. pylori infection on gastric cancer incidence and mortality. In this trial, 3,365 subjects were randomly assigned to either an eradication regimen of amoxicillin and omeprazole or placebo for 2 weeks in 1995. Follow-up examination in 2003 showed H. pylori treatment significantly decreased the combined prevalence of severe chronic atrophic gastritis, intestinal metaplasia, dysplasia or gastric cancer compared with placebo (OR=0.60; 95% CI = 0.47 to 0.75; p<0.001).2Moreover, there were fewer cases of gastric cancer in the eradication group than in the placebo group after 15 years (3.0% vs 4.6%, p=0.032).3
However, Professor El-Omar advised caution with regard to targeting mass eradication of H. pylori as this could lead to potentially negative effects, such as antibiotic resistance and the emergence of acid-related oesophageal diseases. At present, there are still many unanswered questions in gastric cancer prevention: When should eradication happen? How should we eradicate the infection, by mass eradication or by targeting groups at risk? What drugs should we use for eradication? Although emerging evidence shows that gastric cancer seems to be preventable, further research is required to elucidate the best approach to H. pylori eradication.
References
- Helicobacter pylori and Cancer. National Cancer Institute.
http://www.cancer.gov/cancertopics/factsheet/Risk/h-pylori-cancer.
Accessed 8 October 2013. - You WC, et al. J Natl Cancer Inst 2006 19;98:974-983.
- Ma JL, et al. J Natl Cancer Inst 2012;104:488-492.










