New Treatment Strategies for the Eradication of Helicobacter pylori—Combating the Global Trend of Antibiotic Resistance
H. pylori eradication: Efficacy of levofloxacin-based triple therapy as a second-line treatment after triple therapy failure
H. pylori is widely regarded as a significant risk factor for the development of PUD and gastric cancer.1 Successful eradication of this organism is, therefore, essential for preventing these disorders. Notably, the eradication rate of standard triple therapy (ie, clarithromycin, amoxicillin/metronidazole plus a proton pump inhibitor [PPI]) has declined to <80% worldwide in recent years, primarily due to increasing rates of clarithromycin and metronidazole resistance.2-5
Eradication strategies with higher efficacy are urgently needed to improve the H. pylori eradication rate.6 Although regimens containing fluoroquinolones have been used as second-line therapy for H. pylori eradication, there is a lack of trial data to support their use.7
Levofloxacin-based triple therapy:
A viable second-line treatment strategy
Professor Varocha Mahachai and colleagues at Chulalongkorn University and Bangkok Hospital, Thailand, recently led a study to determine the efficacy of levofloxacin-based triple therapy as a second-line treatment strategy in the eradication of H. pylori in patients who had previously failed standard first-line triple therapy.8
The investigators recruited a total of 70 patients (mean age, 53.3 years; 65 patients with gastritis and 5 patients with PUD) who had previously been treated with and had failed a 7.10 day regimen of standard triple therapy, as evaluated by the 13C-Urea Breath Test (UBT). Antral biopsy samples obtained from the subjects by gastroscopy were used for sensitivity tests standard E-test and HelicoDR molecular test9 towards antibiotics such as amoxicillin, clarithromycin and levofloxacin.
Patients were treated with a 10-day regimen of oral levofloxacin (500 mg once daily) plus lansoprazole and amoxicillin (30 mg and 1,000 mg bid, respectively). Eradication of H. pylori was evaluated at 4 weeks post-therapy by 13C-UBT.
Efficacy of levofloxacin-based second-line triple therapy
Approximately 10% of study subjects reported minor side effects but none dropped out. At 4 weeks, the levofloxacin-based triple therapy, as a second-line treatment, resulted in an eradication rate of 62.9% (Figure 1). Patients in this study were considered difficult to treat as they had previously failed first-line standard triple therapy and likely harboured high loads of multiple-drug-resistant organisms. Levofloxacin resistance by HelicoDR test was detected in 50% (18/36) of subjects, and the eradication rate in the levofloxacin-sensitive group was higher than in the levofloxacin-resistant group (66.7% vs 16.7%). The study found no correlation between age or gender and eradication rate.
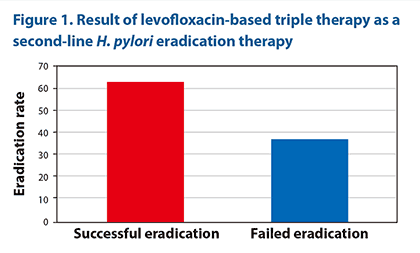 |
| Click on image to enlarge. |
Given that 70% of patients originally achieved H. pylori eradication after first-line standard triple therapy, the 62.9% eradication rate of levofloxacin-based second-line triple therapy in the remaining 30% of patients (who have failed the first-line therapy) led to the cumulative eradication rate of ≈89% [70%+(30%*62.9/100), shown in Figure 2]. Patients who failed both first-and second-line therapies (≈11%) may be subjected to further treatments until permanent cure is achieved.
The investigators concluded that a simple levofloxacin-based triple regimen is effective as second-line therapy for the eradication of H. pylori, with good patient tolerability.
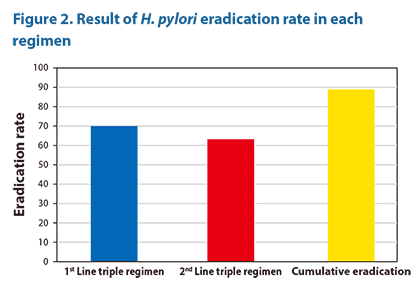 |
| Click on image to enlarge. |
References
- McColl KE. N Engl J Med 2010;362:1597-1604.
- Graham DY, Fischbach L. Gut 2010;59:1143-1153.
- Gisbert JP, et al. Helicobacter 2007;12 Suppl 2:50-58.
- O’Connor A, et al. Helicobacter 2010;15 Suppl 1:46-52.
- O’Connor A, et al. Helicobacter 2011;16 Suppl 1:53-58.
- Gisbert JP, Calvet X. Aliment Pharmacol Ther 2011;34:1255-1268.
- Li Y, et al. Wien Klin Wochenschr 2009;121:47-52.
- Varocha M, et al. Efficacy of levofloxacin-based triple therapy as a second-line therapy after triple therapy failure. Poster session presented at: Gastro 2013 APDW/WCOG, 21.24 September 2013; Shanghai, China.
- Cambau E, et al. J Clin Microbiol 2009 Nov;47(11):3600-3607.










