Optimal Strategic Therapy for Community-Acquired Pneumonia: Levofloxacin at the Forefront of Fluoroquinolone CAP Therapy
Update in Community-Acquired Pneumonia


More than 5.6 million cases of pneumonia are diagnosed annually, with over 55,000 deaths/year in the US alone, emphasizing the need for evidence-based guidelines to optimize management. This article summarizes the current recommendations for treatment of CAP.
The first decision to make after diagnosing CAP is to decide the site of treatment. Prediction rules have been developed to identify whether a patient needs to be hospitalized, including the Pneumonia Severity Index (PSI) and the CURB-65 score [Confusion, Urea > 7 mmol/L, Respiratory rate (RR) ≤ 30 breaths/min, low systolic Blood pressure (SBP) (< 90 mmHg) or diastolic Blood pressure (DBP) (≤ 60 mmHg), and age ≥ 65 years] (1,2). According to CURB-65, a score of 0-1 can be treated as an outpatient, a score of 2 should be hospitalized, and ≥ 3 should be admitted to the intensive care unit (ICU), while PSI classes I and II can be treated as outpatients, with classes IV and V admitted. Class III can be treated either as an outpatient or inpatient.
Consideration then needs to be given to likely pathogens, taking into account sensitivity profiles and changing trends in antibiotic resistance and serotypes. Latest trends in pneumococcal resistance in Asia show that among non-meningeal isolates, penicillin resistance is 0.7%, with 4.6% having intermediate susceptibility, 72.7% are resistant to erythromycin and 59.3% multidrug-resistant (3). Therefore, until a pathogen is identified, relatively broad-spectrum coverage should be given. The risk of having a macrolide-resistant pneumococcal infection should also be considered based on prior antibiotic treatment. If previously treated with a non-macrolide antibiotic, such as a fluoroquinolone or tetracycline, the risk remains low. However, if treated with a macrolide within 3 months, the chance of having a macrolide-resistant pneumococcus is much higher, increasing to > 50% for those treated with azithromycin (4). In contrast, the risk of developing fluoroquinolone-resistant pneumococcus following prior fluoroquinolone therapy is much lower, at < 10% (4).
The Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) 2007 guidelines (5) recommend the following treatment for CAP:
- Outpatients: macrolides or doxycycline for previously healthy patients who have not received antibiotics within 3 months. However, in areas with increasing incidence of resistant pneumococcus, the first option may be a fluoroquinolone.
- Outpatients with co-morbidities or recent antibiotic treatment: respiratory fluoroquinolones, such as levofloxacin, or a β-lactam plus a macrolide. In regions with a high rate of high-level macrolide-resistant Streptococcus pneumoniae, alternative agents should be considered.
- Inpatients, non-ICU: respiratory fluoroquinolone monotherapy or a β-lactam plus a macrolide. However, this latter option is much more nursing time intensive.
- ICU patients: combination therapy with a β-lactam plus either azithromycin or a respiratory fluoroquinolone. For penicillin-allergic patients, a respiratory fluoroquinolone plus aztreonam. If Pseudomonas is a concern, treatment using piperacillin/ tazobactam, cefepime, imipenem, or meropenem together with either levofloxacin or another fluoroquinolone is recommended. Community-acquired methicillin-resistant Staphylococcus aureus is becoming an increasing problem and, in this situation, either linezolid or vancomycin is added.
Latest reports continue to support the IDSA/ATS guidelines, with an important role for fluoroquinolones in managing CAP (6). Levofloxacin has been described as one of the best fluoroquinolones due to its PK/PD features, achieving the highest area under the concentration-time curve (AUC) values compared with ciprofloxacin, moxifloxacin, and gatifloxacin. The level of antibiotic in the epithelial lining fluid (ELF) is also a very good predictor of outcome, with levofloxacin achieving higher ELF concentrations than ciprofloxacin and moxifloxacin.
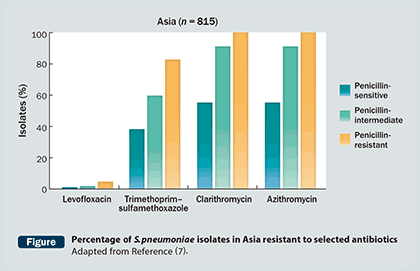
Levofloxacin has also been shown to maintain its efficacy against pneumococcal isolates resistant to other comparators, an important advantage in treating CAP (Figure) (7).
 |
| Click on image to enlarge. |
The pharmacological advantages possessed by levofloxacin have been shown to result in excellent clinical outcomes, with a meta-analysis of hospitalized CAP treatment demonstrating that 6 of 8 studies favor levofloxacin over combination therapy (ceftriaxone plus azithromycin or erythromycin, cefotaxime plus ofloxacin) or versus monotherapy with moxifloxacin (8). A further comparison between levofloxacin and moxifloxacin has been performed focusing on the length of stay (LOS). The results confirmed that levofloxacin 750 mg intravenous (IV) was associated with a much shorter LOS compared with moxifloxacin 400 mg IV (5.83 days for levofloxacin vs. 6.37 days for moxifloxacin, p=0.020) and a reduced cost of therapy (9).
Dr. Mandell concluded that levofloxacin possesses excellent advantages over comparator agents, with oral (PO) and IV administration, excellent bioavailability, and numerous clinical studies supporting its efficacy and safety in CAP.
References
(1) Fine MJ, et al. N Engl J Med 1997; 336: 243-50.
(2) Lim WS, et al. Thorax 2003; 58: 377-82.
(3) Kim SH, et al. Antimicrob Agents Chemother 2012; 56: 1418-26.
(4) Vanderkooi OG, et al. Clin Infect Dis 2005; 40: 1288-97.
(5) Mandell LA, et al. Clin Infect Dis 2007; 44 Suppl 2: S27-72.
(6) Wunderink RG, et al. N Engl J Med 2014; 370: 543-51.
(7) Global Landscape On the Bactericidal Activity of Levofloxacin (GLOBAL) surveillance study 2005/2006.
(8) Salkind AR, et al. Ann Pharmacother 2002; 36: 1938-43.
(9) Schein J, et al. Curr Med Res Opin 2008; 24: 895-906.









