Levofloxacin: a Mainstay of Fluoroquinolone-Based Therapy-After Twenty Years of Use, Levofloxacin Remains Safe and Effective
Reviewing Levofloxacin Safety: Levofloxacin Maintains a Favorable Risk-Benefit Profile
Quinolones were first introduced in the 1960s, and since that time, they have undergone numerous structural modifications to become mainstays of antimicrobial therapy. Over their long history of use, safety concerns have been raised, with some adverse drug reactions (ADRs) reported as class effects while others remain linked to individual agents. However, during the past 20 years, levofloxacin has continued to be used extensively and, unlike many other comparator fluoroquinolones, has been shown to maintain efficacy, while at the same time providing a positive risk-benefit ratio. Recently, additional issues relating to safety have been raised, particularly relating to hepatic and renal problems. To discuss the validity of these issues, a panel of international experts came together to review and update the safety data relating to levofloxacin, with presentations by Prof. Rafael Cantón, Spain who reported on resistance issues, followed by Emeritus Prof. Lionel A. Mandell, Canada, who reviewed the use of levofloxacin in the respiratory setting, and the moderator of the session, Prof. Kurt G. Naber, Germany, who outlined the use of levofloxacin in urology.
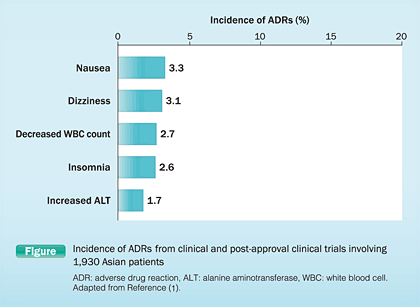
Levofloxacin has been used continuously in Japan since it was first launched in 1993 with the 7th Japanese updated package insert revised in November 2011. This reported that the main ADRs for levofloxacin in Japan and China from clinical and post-approval clinical trials of 1,930 patients were similar to those reported as fluoroquinolone-class effects, namely, nausea (3.3%), dizziness (3.1%), decreased white blood cell (WBC) count (2.7%), insomnia (2.6%), and increased alanine aminotransferase (ALT) (1.7%) (Figure) (1).
 |
| Click on image to enlarge. |
Supporting the safety of levofloxacin are results from a large post-marketing study conducted from October 2009 to September 2010, which reported no significant safety problems. Gastrointestinal disorders were reported in 0.64%, abnormal laboratory findings in 0.31%, and skin and subcutaneous tissue disorders in 0.19%. The overall ADR rate was 1.6% (482 out of 29,880 patients) (1).
Levofloxacin has been used extensively worldwide, with a very long-established track record. It has been marketed in 124 countries with more than 851 million total prescriptions. This extensive database provides clear evidence supporting the use of levofloxacin in many different patient groups and its safety and efficacy have been confirmed with global practice guidelines. This post-marketing surveillance is ongoing, providing an invaluable and growing database of any potential ADRs, allowing physicians to feel secure about the relative safety of this very useful agent.
Reference
(1) Hori S, et al. Jpn J Chemother 2011; 59: 614-33.










