Optimizing the Management of Prostatitis: Levofloxacin and Silodosin—Strategies Aimed at Optimal Treatment for Prostatitis
Role of α1-Blockers in Chronic Prostatitis Syndromes: A History of Progressive Research


CP syndromes are difficult-to-treat urological disorders associated with genitourinary pain, obstructive or irritative voiding issues and a reduced quality of life (QOL). A number of therapeutic modalities have been used to manage these conditions including α-blockers, with the first uncontrolled trials using non-validated outcome measures investigating these agents in CP/CPPS demonstrating a possible beneficial effect (1, 2). A network meta-analysis incorporating all relevant contemporary clinical trials has confirmed that these agents are better than antibiotics and placebo, but only modestly (3).
Further investigation has differentiated between specific α-antagonists. One agent of growing interest is silodosin, a highly selective α1A-adrenergic receptor antagonist approved by the FDA in 2008 for lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH) based on 2 pivotal trials that demonstrated it to be very safe and effective (4, 5).
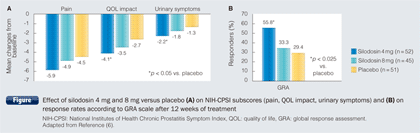
In 2011, silodosin was also approved in China for the treatment of the signs and symptoms of BPH. Prof. Nickel reported it to be both effective and safe. He also reported data showing that silodosin once daily in patients with moderate to severe abacterial CP/CPPS was associated with a significantly greater decrease in total National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) score as compared with placebo (-12.1 vs. -8.5, p = 0.0224). Further subgroup analysis also demonstrated that 4 mg silodosin achieved significantly greater improvement in urinary symptoms (-2.2 vs. -1.3, p < 0.05) and QOL impact (-4.1 vs. -2.7, p < 0.05) compared with placebo (Figure A) (6). In fact, the percentage of patients with moderate-marked global improvement at the end of treatment measured according to the global response assessment (GRA) scale was almost twice as high with silodosin 4 mg compared with placebo (55.8% vs. 29.4%, p < 0.025) (Figure B) (6). In terms of safety, the incidence of drug-related adverse events for silodosin 4 mg and placebo were similar, apart from the incidence of retrograde ejaculation.
 |
| Click on image to enlarge. |
A recent updated meta-analysis confirmed that treatment with α-blockers, possibly in combination with antimicrobials is effective in relieving CPPS symptoms, and third generation agents, such as silodosin and tamsulosin, may be associated with clinical efficacy with reduced cardiovascular side effects (7).
These results have led to the 2012 International Consultation on Urological Diseases (ICUD) recommending α-blockers, such as silodosin, for treating newly diagnosed α-blocker naïve patients with voiding symptoms and multimodal therapy directed according to clinical phenotype. However, α-blocker monotherapy is not recommended for patients with voiding symptoms or those who have previously failed treatment with α-blockers.
References
(1) Neal DE Jr, et al. Urology 1994; 43: 460-5.
(2) Barbalias GA, et al. J Urol 1998; 159: 883-7.
(3) Anothaisintawee T, et al. JAMA 2011; 305: 78-86.
(4) Marks LS, et al. J Urol 2009; 181: 2634-40.
(5) Marks LS, et al. Urology 2009; 74: 1318-22.
(6) Nickel JC, et al. J Urol 2011; 186: 125-31.
(7) Nickel JC, et al. Rev Urol 2012; 14: 56-64.










