The Treatment of Community-Acquired Pneumonia with Consideration of Mycobacterium Tuberculosis
Present and Future Use of Fluoroquinolones in Tuberculosis-Issues of Disease Masking and Bacillary Resistance


TB is a significant global problem, with multidrug-resistant and extensively drug-resistant forms posing a formidable challenge to its control. Fluoroquinolones, well known for their efficacy in treating RTIs, also play a role in treating MDR-TB, and drug-susceptible TB, especially in the presence of severe adverse reactions to first-line conventional drugs. In addition, they have the potential to prevent the spread of MDR-TB and, possibly, shorten the total duration of therapy. Prof. Wing Wai Yew, The Chinese University of Hong Kong, China, reviewed the role of fluoroquinolones in TB, giving particular attention to the issue of disease masking and the development of resistance.
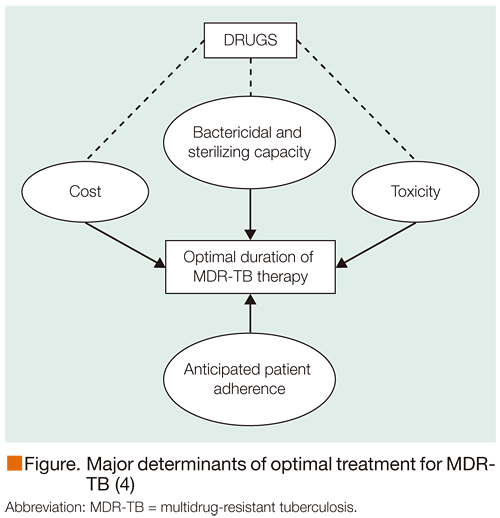
Fluoroquinolones are regarded as the most potent second-line drugs for the treatment of MDR-TB, with bacillary resistance to these agents associated with a worse outcome (1). In addition, recent data have highlighted the high mortality associated with extensively drug-resistant TB (XDR-TB), but noted that treatment with newer-generation fluoroquinolones is an independent predictor of survival (2). Further support for fluoroquinolones in XDR-TB is provided by a meta-analysis showing that patients treated with newer fluoroquinolones report a higher proportion of favorable treatment outcomes, despite susceptibility testing revealing in vitrofluoroquinolone resistance (3). The major determinants of optimal treatment duration for MDR-TB include the bactericidal and sterilizing capacity plus the drug cost and toxicity and anticipation of adherence of patient (Figure) (4).
Major new fluoroquinolones available include levofloxacin and moxifloxacin. A study investigating the early and extended bactericidal activity of levofloxacin 1,000 mg daily found it to be better than moxifloxacin 400 mg daily and equivalent to isoniazid 300 mg daily (5). While higher doses of moxifloxacin are likely to achieve excellent bactericidal activity against M. tuberculosis and to suppress drug resistance, the tolerability of these higher doses is unknown (6). In contrast, levofloxacin has a well-documented safety record including its use in patients with hepatic dysfunction, a useful feature in managing TB in patients with hepatotoxicity (7).
Prof. Yew noted that if fluoroquinolones are to maintain their efficacy, it is important that they are prescribed with great care. This raises the question of whether empiric treatment of CAP with fluoroquinolones delays the treatment of TB and increases the development of resistance. A randomized study investigating this found that 4.8% of patients on amoxicillin-clavulanic acid developed active TB compared with 1.4% on a fluoroquinolone after their use in treating lower RTIs, with the difference being significant in terms of both the proportion and time-to-event analyses (8). Post-hoc analysis showed a significant decrease in the proportion with active TB from 4.8% in the amoxicillin-clavulanic acid group to 2.4% in the 5-day fluoroquinolone group and 0% in the 10-day fluoroquinolone group, indicating that newer fluoroquinolones can mask active TB. In addition, the longer the fluoroquinolone is used, the higher the masking effect.
Prof. Yew reported further data supporting the masking effect of fluoroquinolones, with results from an important meta-analysis showing that diagnosis and treatment of pulmonary TB was significantly delayed by an average of 19.03 days in patients treated with fluoroquinolones (9). Moreover, the pooled odds ratio of developing a fluoroquinolone-resistant M. tuberculosis strain was 2.70 (95% confidence interval; 1.30-5.60). This trend was confirmed by results from a study showing that patients recently exposed to fluoroquinolones for 5 days or more were less likely to be M. tuberculosis smear-positive, resulting in an increased time to treatment (10). Turning to the issue of resistance, Prof. Yew described results showing that fluoroquinolone resistance in M. tuberculosis has been identified as early as 13 days after initial therapy, although he noted that the subjects were human immunodeficiency virus (HIV)-positive and advanced immunosuppression may have contributed to the development of this resistance (11). Another study evaluating the effect of the duration and timing of fluoroquinolone exposure controlled for age, sex, race, HIV status and the site of the disease found that fluoroquinolone exposure for more than 10 days, occurring more than 60 days before diagnosis of TB, was associated with the highest risk of fluoroquinolone resistance (12).
Countering this were results demonstrating that patients prescribed a single fluoroquinolone course were not at increased risk of developing resistance, although patients who had received multiple fluoroquinolone prescriptions were more likely to have fluoroquinolone-resistant TB (13). Furthermore, Prof. Yew reported that resistance to M. tuberculosis mainly results from suboptimal M. tuberculosis treatment rather than the widespread use of fluoroquinolones in bacterial infections, in many countries (14).
Analysis of independent factors for fluoroquinolone-resistant M. tuberculosis identified the following as risks: single, migrants, retreatment patients, previous exposure to fluoroquinolones, chronic obstructive pulmonary disease, MDR-TB or XDR-TB in a center in China (15). Therefore, fluoroquinolone-resistant M. tuberculosis probably developed here through a possible combination of two mechanisms namely long-term use in MDR-TB and short-term use in bacterial infections, especially when recurrent courses of long durations were prescribed.
Prof. Yew commented that any strategy to forestall the development of M. tuberculosis resistance when treating CAP must first achieve a balance between the benefits of fluoroquinolone treatment in CAP and the risks of masking/resistance in TB. It is important to remember that consensus guidelines on the management of CAP recommend fluoroquinolones, especially in difficult patients with comorbidities such as chronic heart, lung, hepatic and renal diseases, diabetes mellitus, alcoholism, malignancies, and immunosuppression. However, while these CAP patients are likely to benefit from fluoroquinolones, they are also the same group that are at increased risk of having TB. Therefore, the clinician must ask what is the likelihood of TB as a primary or concomitant pathology, also taking into consideration host risk factors and radiographic appearance. Prof. Yew recommended that if there is a significant risk of TB, rapid diagnostic testing including genotypic tests should be performed. He also stressed the need for clinicians to be acquainted with the radiographic features of TB, as a retrospective analysis has shown that 94% of TB cases treated as pneumonia, had an initial chest X-ray suggestive of TB, with many of these apparently overlooked.
Proposed measures to forestall development of fluoroquinolone-resistant TB when treating CAP include limiting the duration of fluoroquinolone treatment to no more than 5 to 7 days, avoiding recurrent courses of fluoroquinolones and preferring the use of high daily doses of potent agents like levofloxacin. In addition, when an etiological pathogen has been identified, Prof. Yew said that clinicians should use the most appropriate antibiotic, changing to an alternative if warranted from the clinical progress. This is particularly relevant for suspected pathogens, such as H. influenzae, Moraxella catarrhalis and S. aureus, but if atypical pathogens or PRSP are suspected, empiric fluoroquinolones should still be used but with great care. Prof. Yew finished by stressing the need for achieving a balance between the benefits of fluoroquinolones in CAP and the potential risks in TB, remembering that a short-course regimen of a respiratory fluoroquinolone can still be recommended for empiric therapy in CAP if the patient is at low risk for TB.
References
(1) Yew WW, et al. Chest 2000; 117(3): 744-51.
(2) Dheda K, et al. Lancet 2010; 375(9728): 1798-807.
(3) Jacobson KR, et al. Clin Infect Dis 2010; 51(1): 6-14.
(4) Nuermberger EL, et al. Respirology 2010; 15(5): 764-78.
(5) Johnson JL, et al. Int J Tuberc Lung Dis 2006; 10(6): 605-12.
(6) Gumbo T, et al. J Infect Dis 2004; 190(9): 1642-51.
(7) Carbon C. Chemotherapy 2001; 47 Suppl 3: 9-14.
(8) Chang KC, et al. Eur Respir J 2010; 35(3): 606-13.
(9) Chen TC, et al. Int J Infect Dis 2011; 15(3): e211-6.
(10) Jeon CY, et al. Int J Tuberc Lung Dis 2011; 15(1): 77-83.
(11) Ginsburg AS, et al. N Engl J Med 2003; 349(20): 1977-8.
(12) Devasia RA, et al. Am J Respir Crit Care Med 2009; 180(4): 365-70.
(13) Long R, et al. Clin Infect Dis 2009; 48(10): 1354-60.
(14) Huang TS, et al. J Antimicrob Chemother 2005; 56(6): 1058-62.
(15) Liu CH, et al. Respirology 2011; 16(6): 918-25.









